Synapsin I Controls Synaptic Maturation of Long-Range Projections in the Lateral Amygdala in a Targeted Selective Fashion
- PMID: 31164805
- PMCID: PMC6536628
- DOI: 10.3389/fncel.2019.00220
Synapsin I Controls Synaptic Maturation of Long-Range Projections in the Lateral Amygdala in a Targeted Selective Fashion
Abstract
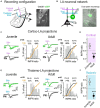
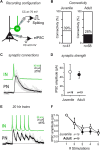
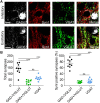
The amygdala, and more precisely its lateral nucleus, is thought to attribute emotional valence to external stimuli by generating long-term plasticity changes at long-range projections to principal cells. Aversive experience has also been shown to modify pre- and post-synaptic markers in the amygdala, suggesting their possible role in the structural organization of adult amygdala networks. Here, we focused on how the maturation of cortical and thalamic long-range projections occurs on principal neurons and interneurons in the lateral amygdala (LA). We performed dual electrophysiological recordings of identified cells in juvenile and adult GAD67-GFP mice after independent stimulation of cortical and thalamic afferent systems. The results demonstrate that synaptic strengthening occurs during development at synapses projecting to LA principal neurons, but not interneurons. As synaptic strengthening underlies fear conditioning which depends, in turn, on presence and increasing expression of synapsin I, we tested if synapsin I contributes to synaptic strengthening during development. Interestingly, the physiological synaptic strengthening of cortical and thalamic synapses projecting to LA principal neurons was virtually abolished in synapsin I knockout mice, but not differences were observed in the excitatory projections to interneurons. Immunohistochemistry analysis showed that the presence of synapsin I is restricted to excitatory contacts projecting to principal neurons in LA of adult mice. These results indicate that synapsin I is a key regulator of the maturation of synaptic connectivity in this brain region and that is expression is dependent on postsynaptic identity.
Keywords: amygdala; cortical projections; interneurons; synapse maturation; synapsin; thalamic projections.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

