The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy
- PMID: 31164885
- PMCID: PMC6536589
- DOI: 10.3389/fimmu.2019.01074
The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy
Abstract
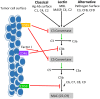
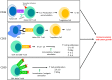
It has long been understood that the control and surveillance of tumors within the body involves an intricate dance between the adaptive and innate immune systems. At the center of the interplay between the adaptive and innate immune response sits the complement system-an evolutionarily ancient response that aids in the destruction of microorganisms and damaged cells, including cancer cells. Membrane-bound complement regulatory proteins (mCRPs), such as CD46, CD55, and CD59, are expressed throughout the body in order to prevent over-activation of the complement system. These mCRPs act as a double-edged sword however, as they can also over-regulate the complement system to the extent that it is no longer effective at eliminating cancerous cells. Recent studies are now indicating that mCRPs may function as a biomarker of a malignant transformation in numerous cancer types, and further, are being shown to interfere with anti-tumor treatments. This highlights the critical roles that therapeutic blockade of mCRPs can play in cancer treatment. Furthermore, with the complement system having the ability to both directly and indirectly control adaptive T-cell responses, the use of a combinatorial approach of complement-related therapy along with other T-cell activating therapies becomes a logical approach to treatment. This review will highlight the biomarker-related role that mCRP expression may have in the classification of tumor phenotype and predicted response to different anti-cancer treatments in the context of an emerging understanding that complement activation within the Tumor Microenvironment (TME) is actually harmful for tumor control. We will discuss what is known about complement activation and mCRPs relating to cancer and immunotherapy, and will examine the potential for combinatorial approaches of anti-mCRP therapy with other anti-tumor therapies, especially checkpoint inhibitors such as anti PD-1 and PD-L1 monoclonal antibodies (mAbs). Overall, mCRPs play an essential role in the immune response to tumors, and understanding their role in the immune response, particularly in modulating currently used cancer therapeutics may lead to better clinical outcomes in patients with diverse cancer types.
Keywords: combination therapy; complement cascade; immunotherapy; mCRP; oncology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

