A Fast Transient Absorption Study of Co(AcAc)3
- PMID: 31165061
- PMCID: PMC6536591
- DOI: 10.3389/fchem.2019.00348
A Fast Transient Absorption Study of Co(AcAc)3
Abstract
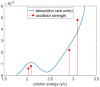
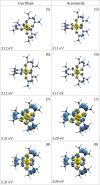
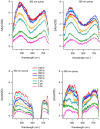
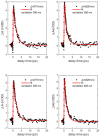
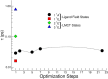
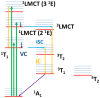
The study of transition metal coordination complexes has played a key role in establishing quantum chemistry concepts such as that of ligand field theory. Furthermore, the study of the dynamics of their excited states is of primary importance in determining the de-excitation path of electrons to tailor the electronic properties required for important technological applications. This work focuses on femtosecond transient absorption spectroscopy of Cobalt tris(acetylacetonate) (Co(AcAc)3) in solution. The fast transient absorption spectroscopy has been employed to study the excited state dynamics after optical excitation. Density functional theory coupled with the polarizable continuum model has been used to characterize the geometries and the electronic states of the solvated ion. The excited states have been calculated using the time dependent density functional theory formalism. The time resolved dynamics of the ligand to metal charge transfer excitation revealed a biphasic behavior with an ultrafast rise time of 0.07 ± 0.04 ps and a decay time of 1.5 ± 0.3 ps, while the ligand field excitations dynamics is characterized by a rise time of 0.07 ± 0.04 ps and a decay time of 1.8 ± 0.3 ps. Time dependent density functional theory calculations of the spin-orbit coupling suggest that the ultrafast rise time can be related to the intersystem crossing from the originally photoexcited state. The picosecond decay is faster than that of similar cobalt coordination complexes and is mainly assigned to internal conversion within the triplet state manifold. The lack of detectable long living states (>5 ps) suggests that non-radiative decay plays an important role in the dynamics of these molecules.
Keywords: TDDFT (time-dependent density functional theory) calculations; charge - transfer; fast transient absorption; femtosecand laser pulses; metal complexes.
Figures





References
-
- ADF (2018). SCM, Theoretical Chemistry, eds Baerends E. J., Ziegler T., Atkins A. J., Autschbach J., Baseggio O., Bashford D., et al. (Amsterdam: Vrije Universiteit; ). Available online at: http://www.scm.com
-
- Bauernschmitt R., Ahlrichs R. (1996). Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 256, 454–464. 10.1016/0009-2614(96)00440-X - DOI
-
- Becke A. D. (1993). Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652. 10.1063/1.464913 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

