Consensus Paper: Experimental Neurostimulation of the Cerebellum
- PMID: 31165428
- PMCID: PMC6867990
- DOI: 10.1007/s12311-019-01041-5
Consensus Paper: Experimental Neurostimulation of the Cerebellum
Abstract
The cerebellum is best known for its role in controlling motor behaviors. However, recent work supports the view that it also influences non-motor behaviors. The contribution of the cerebellum towards different brain functions is underscored by its involvement in a diverse and increasing number of neurological and neuropsychiatric conditions including ataxia, dystonia, essential tremor, Parkinson's disease (PD), epilepsy, stroke, multiple sclerosis, autism spectrum disorders, dyslexia, attention deficit hyperactivity disorder (ADHD), and schizophrenia. Although there are no cures for these conditions, cerebellar stimulation is quickly gaining attention for symptomatic alleviation, as cerebellar circuitry has arisen as a promising target for invasive and non-invasive neuromodulation. This consensus paper brings together experts from the fields of neurophysiology, neurology, and neurosurgery to discuss recent efforts in using the cerebellum as a therapeutic intervention. We report on the most advanced techniques for manipulating cerebellar circuits in humans and animal models and define key hurdles and questions for moving forward.
Keywords: Cerebellum; DBS; Neuromodulation; Neurostimulation; Non-invasive therapy; Optogenetics.
Conflict of interest statement
LN Miterko, J Beckinghausen, RV Sillitoe: None
LV Bradnam, AB McCambridge: None
J Cooperrider, KB Baker, A Machado: None
M Hallett: Member of the scientific advisory board of Brainsway T Popa: None
DH Heck: None
T Wichmann, MR DeLong: None
SV Gornati, FE Hoebeek: None
AZ Kouzani: None
S-H Kuo, T Xie, ED Louis: None
M Manto, N Oulad Ben Taib: None
MA Nitsche: Member of the scientific advisory board of Neuroelectrics
M Tanaka: None
D Timmann: None
MY Cheng, EH Wang, GK Steinberg: None
Figures
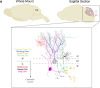

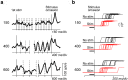
References
-
- Ramón y Cajal S. Nerf trigemeau ou de la Ve paire. Maloine. Histol. du Syst. Nerv. L’Homme des vertébrés. Paris; 1909.
-
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Cogn Sci. 1998;21:370–375. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS100874/NS/NINDS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K08 NS083738/NS/NINDS NIH HHS/United States
- R01-NS062876/NH/NIH HHS/United States
- P50-NS098685/NH/NIH HHS/United States
- R01 NS116384/NS/NINDS NIH HHS/United States
- R01NS089664/NS/NINDS NIH HHS/United States
- R01 NS088257/NS/NINDS NIH HHS/United States
- R01 MH112143/MH/NIMH NIH HHS/United States
- R01 NS094607/NS/NINDS NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- R01 NS105899/NS/NINDS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- R01 NS073872/NS/NINDS NIH HHS/United States
- R01 NS085136/NS/NINDS NIH HHS/United States
- P50 NS098685/NS/NINDS NIH HHS/United States
- R01 NS089664/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

