Permeability barriers of Gram-negative pathogens
- PMID: 31165502
- PMCID: PMC6940542
- DOI: 10.1111/nyas.14134
Permeability barriers of Gram-negative pathogens
Abstract
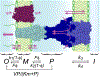
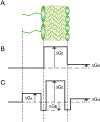
Most clinical antibiotics do not have efficacy against Gram-negative pathogens, mainly because these cells are protected by the permeability barrier comprising the two membranes with active efflux. The emergence of multidrug-resistant Gram-negative strains threatens the utility even of last resort therapeutic treatments. Significant efforts at different levels of resolution are currently focused on finding a solution to this nonpermeation problem and developing new approaches to the optimization of drug activities against multidrug-resistant pathogens. The exceptional efficiency of the Gram-negative permeability barrier is the result of a complex interplay between the two opposing fluxes of drugs across the two membranes. In this review, we describe the current state of understanding of the problem and the recent advances in theoretical and empirical approaches to characterization of drug permeation and active efflux in Gram-negative bacteria.
Keywords: Gram-negative; antibiotic resistance; bacteria; multidrug efflux; permeability barrier.
© 2019 New York Academy of Sciences.
Conflict of interest statement
Figures
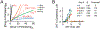

References
-
- Tommasi R; Brown DG; Walkup GK; Manchester JI; Miller AA, ESKAPEing the labyrinth of antibacterial discovery. Nat Rev Drug Discov 2015, 14 (8), 529–42. - PubMed
-
- Boucher HW; Scheld M; Bartlett J; Talbot GH; Bradley JS; Spellberg B; Edwards JE; Gilbert D; Rice LB, Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clinical Infectious Diseases 2009, 48 (1), 1–12. - PubMed
-
- Nikaido H, Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 1994, 264 (5157), 382–388. - PubMed
-
- Borneleit P; Kleber H-P, The outer membrane of Acinetobacter: structure-function relationships In The Biology of Acinetobacter, Towner KJ; Bergogne-Berezin E; Fewson CA, Eds. Plenum Press: New York and London, 1991; pp 259–272.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

