Metformin treatment reduces motor and neuropsychiatric phenotypes in the zQ175 mouse model of Huntington disease
- PMID: 31165723
- PMCID: PMC6549163
- DOI: 10.1038/s12276-019-0264-9
Metformin treatment reduces motor and neuropsychiatric phenotypes in the zQ175 mouse model of Huntington disease
Abstract
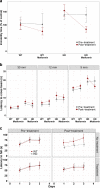
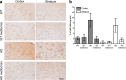
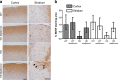
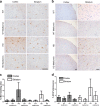
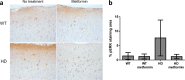
Huntington disease is a neurodegenerative condition for which there is no cure to date. Activation of AMP-activated protein kinase has previously been shown to be beneficial in in vitro and in vivo models of Huntington's disease. Moreover, a recent cross-sectional study demonstrated that treatment with metformin, a well-known activator of this enzyme, is associated with better cognitive scores in patients with this disease. We performed a preclinical study using metformin to treat phenotypes of the zQ175 mouse model of Huntington disease. We evaluated behavior (motor and neuropsychiatric function) and molecular phenotypes (aggregation of mutant huntingtin, levels of brain-derived neurotrophic factor, neuronal inflammation, etc.). We also used two models of polyglutamine toxicity in Caenorhabditis elegans to further explore potential mechanisms of metformin action. Our results provide strong evidence that metformin alleviates motor and neuropsychiatric phenotypes in zQ175 mice. Moreover, metformin intake reduces the number of nuclear aggregates of mutant huntingtin in the striatum. The expression of brain-derived neurotrophic factor, which is reduced in mutant animals, is partially restored in metformin-treated mice, and glial activation in mutant mice is reduced in metformin-treated animals. In addition, using worm models of polyglutamine toxicity, we demonstrate that metformin reduces polyglutamine aggregates and restores neuronal function through mechanisms involving AMP-activated protein kinase and lysosomal function. Our data indicate that metformin alleviates the progression of the disease and further supports AMP-activated protein kinase as a druggable target against Huntington's disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Ferrante RJ, Kowall NW, Richardson EP., Jr Proliferative and degenerative changes in striatal spiny neurons in Huntington's disease: a combined study using the section-Golgi method and calbindin D28k immunocytochemistry. J. Neurosci. 1991;11:3877–3887. doi: 10.1523/JNEUROSCI.11-12-03877.1991. - DOI - PMC - PubMed

