Spatiotemporal coordination of trophoblast and allantoic Rbpj signaling directs normal placental morphogenesis
- PMID: 31165749
- PMCID: PMC6549187
- DOI: 10.1038/s41419-019-1683-1
Spatiotemporal coordination of trophoblast and allantoic Rbpj signaling directs normal placental morphogenesis
Abstract
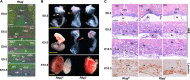
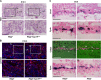
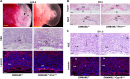
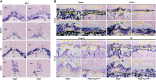
The placenta, responsible for the nutrient and gas exchange between the mother and fetus, is pivotal for successful pregnancy. It has been shown that Rbpj, the core transcriptional mediator of Notch signaling pathway, is required for normal placentation in mice. However, it remains largely unclear how Rbpj signaling in different placental compartments coordinates with other important regulators to ensure normal placental morphogenesis. In this study, we found that systemic deletion of Rbpj led to abnormal chorioallantoic morphogenesis and defective trophoblast differentiation in the ectoplacental cone (EPC). Employing mouse models with selective deletion of Rbpj in the allantois versus trophoblast, combining tetraploid aggregation assay, we demonstrated that allantois-expressed Rbpj is essential for chorioallantoic attachment and subsequent invagination of allantoic blood vessels into the chorionic ectoderm. Further studies uncovered that allantoic Rbpj regulates chorioallantoic fusion and morphogenesis via targeting Vcam1 in a Notch-dependent manner. Meanwhile, we also revealed that trophoblast-expressed Rbpj in EPC facilitates Mash2's transcriptional activity, promoting the specification of Tpbpα-positive trophoblasts, which differentiate into trophoblast subtypes responsible for interstitial and endovascular invasion at the later stage of placental development. Collectively, our study further shed light on the molecular network governing placental development and functions, highlighting the necessity of a spatiotemporal coordination of Rbpj signaling for normal placental morphogenesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

