Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation
- PMID: 31166007
- PMCID: PMC6549450
- DOI: 10.1002/14651858.CD009670.pub4
Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation
Abstract
Background: Pharmacotherapies for smoking cessation increase the likelihood of achieving abstinence in a quit attempt. It is plausible that providing support, or, if support is offered, offering more intensive support or support including particular components may increase abstinence further.
Objectives: To evaluate the effect of adding or increasing the intensity of behavioural support for people using smoking cessation medications, and to assess whether there are different effects depending on the type of pharmacotherapy, or the amount of support in each condition. We also looked at studies which directly compare behavioural interventions matched for contact time, where pharmacotherapy is provided to both groups (e.g. tests of different components or approaches to behavioural support as an adjunct to pharmacotherapy).
Search methods: We searched the Cochrane Tobacco Addiction Group Specialised Register, clinicaltrials.gov, and the ICTRP in June 2018 for records with any mention of pharmacotherapy, including any type of nicotine replacement therapy (NRT), bupropion, nortriptyline or varenicline, that evaluated the addition of personal support or compared two or more intensities of behavioural support.
Selection criteria: Randomised or quasi-randomised controlled trials in which all participants received pharmacotherapy for smoking cessation and conditions differed by the amount or type of behavioural support. The intervention condition had to involve person-to-person contact (defined as face-to-face or telephone). The control condition could receive less intensive personal contact, a different type of personal contact, written information, or no behavioural support at all. We excluded trials recruiting only pregnant women and trials which did not set out to assess smoking cessation at six months or longer.
Data collection and analysis: For this update, screening and data extraction followed standard Cochrane methods. The main outcome measure was abstinence from smoking after at least six months of follow-up. We used the most rigorous definition of abstinence for each trial, and biochemically-validated rates, if available. We calculated the risk ratio (RR) and 95% confidence interval (CI) for each study. Where appropriate, we performed meta-analysis using a random-effects model.
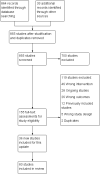
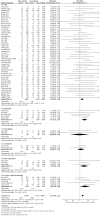
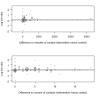
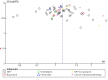
Main results: Eighty-three studies, 36 of which were new to this update, met the inclusion criteria, representing 29,536 participants. Overall, we judged 16 studies to be at low risk of bias and 21 studies to be at high risk of bias. All other studies were judged to be at unclear risk of bias. Results were not sensitive to the exclusion of studies at high risk of bias. We pooled all studies comparing more versus less support in the main analysis. Findings demonstrated a benefit of behavioural support in addition to pharmacotherapy. When all studies of additional behavioural therapy were pooled, there was evidence of a statistically significant benefit from additional support (RR 1.15, 95% CI 1.08 to 1.22, I² = 8%, 65 studies, n = 23,331) for abstinence at longest follow-up, and this effect was not different when we compared subgroups by type of pharmacotherapy or intensity of contact. This effect was similar in the subgroup of eight studies in which the control group received no behavioural support (RR 1.20, 95% CI 1.02 to 1.43, I² = 20%, n = 4,018). Seventeen studies compared interventions matched for contact time but that differed in terms of the behavioural components or approaches employed. Of the 15 comparisons, all had small numbers of participants and events. Only one detected a statistically significant effect, favouring a health education approach (which the authors described as standard counselling containing information and advice) over motivational interviewing approach (RR 0.56, 95% CI 0.33 to 0.94, n = 378).
Authors' conclusions: There is high-certainty evidence that providing behavioural support in person or via telephone for people using pharmacotherapy to stop smoking increases quit rates. Increasing the amount of behavioural support is likely to increase the chance of success by about 10% to 20%, based on a pooled estimate from 65 trials. Subgroup analysis suggests that the incremental benefit from more support is similar over a range of levels of baseline support. More research is needed to assess the effectiveness of specific components that comprise behavioural support.
Conflict of interest statement
JHB: none known
BH: none known
JLB: none known
HW: none known
TRF: none known
Figures





Update of
-
Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation.Cochrane Database Syst Rev. 2015 Oct 12;(10):CD009670. doi: 10.1002/14651858.CD009670.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jun 05;6:CD009670. doi: 10.1002/14651858.CD009670.pub4. PMID: 26457723 Updated.
References
References to studies included in this review
Ahluwalia 2006 {published data only}
-
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction 2006;101(6):883‐91. - PubMed
-
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 2007;102(12):1979‐86. - PubMed
Aimer 2017 {published data only}
-
- Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis‐specific smoking cessation programme; a pilot randomized controlled trial. Arthritis Care & Research 2017;69(1):28‐37. - PubMed
Alterman 2001 {published data only}
-
- Alterman AI, Gariti P, Cook TG, Cnaan A. Nicodermal patch adherence and its correlates. Drug & Alcohol Dependence 1999;53(2):159‐65. - PubMed
-
- Alterman AI, Gariti P, Mulvaney F. Short‐ and long‐term smoking cessation for three levels of intensity of behavioral treatment. Psychology of Addictive Behaviors 2001;15(3):261‐4. - PubMed
Aveyard 2007 {published data only}
-
- Greaves C. Smoking cessation trial may be missing the point [comment]. Thorax 2008;63(3):291‐2. - PubMed
Bailey 2013 {published data only}
Baker 2015 {published data only}
-
- ACTRN12609001039279. Healthy lifestyle intervention for cardiovascular disease risk reduction among smokers with psychotic disorders. Australia and New Zealand Clinical Trials Registry (first received 4 December 2009).
-
- Baker AL, Richmond R, Kay‐Lambkin FJ, Filia SL, Castle D, Williams JM, et al. Randomised controlled trial of a healthy lifestyle intervention among smokers with psychotic disorders: outcomes to 36 months. Australian and New Zealand Journal of Psychiatry 2018;52(3):239‐52. - PubMed
-
- Baker AL, Richmond R, Kay‐Lambkin FJ, Filia SL, Castle D, Williams JM, et al. Randomized controlled trial of a healthy lifestyle intervention among smokers with psychotic disorders. Nicotine & Tobacco Research 2015;17(8):946‐54. - PubMed
Bastian 2012 {published data only}
-
- Bastian LA, Fish LJ, Gierisch JM, Rohrer LD, Stechuchak KM, Grambow SC. Comparative effectiveness trial of family‐supported smoking cessation intervention versus standard telephone counseling for chronically ill veterans using proactive recruitment. Comparative Effectiveness Research 2012;2:45‐56.
-
- NCT00448344. Family‐supported smoking cessation for chronically ill veterans. ClinicalTrials.gov/show/NCT00448344 (first received 16 March 2007).
Begh 2015 {published data only}
Berndt 2014 {published data only}
-
- Berndt N, Bolman C, Froelicher ES, Mudde A, Candel M, Vries H, et al. Effectiveness of a telephone delivered and a face‐to‐face delivered counseling intervention for smoking cessation in patients with coronary heart disease: a 6‐month follow‐up. Journal of Behavioral Medicine 2014;37(4):709‐24. [CENTRAL: 1000936; CRS: 9400107000001638; PUBMED: 23760610] - PubMed
-
- Berndt N, Bolman C, Lechner L, Mudde A, Verheugt FW, Vries H. Effectiveness of two intensive treatment methods for smoking cessation and relapse prevention in patients with coronary heart disease: study protocol and baseline description. BMC Cardiovascular Disorders 2012;12:33. [CENTRAL: 840357; CRS: 9400123000016156; EMBASE: 2012574240; PUBMED: 22587684] - PMC - PubMed
-
- Berndt N, Lechner L, Vries H, Acker F, Mudde A, Froelicher E, et al. Effectiveness of two intensive smoking cessation interventions for the secondary prevention of coronary heart disease: benefits for patients with low socioeconomic status and low quit motivations. European Heart Journal 2013;34:475. [CENTRAL: 1006097; CRS: 9400050000000077; EMBASE: 71259288]
Bloom 2017 {published data only}
Bock 2014 {published data only}
-
- Bock BC, Papandonatos GD, Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low‐income smokers: motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research 2014;16(4):413‐22. [CENTRAL: 986341; CRS: 9400050000000050; EMBASE: 2014185069; PUBMED: 24174612] - PMC - PubMed
Boyle 2007 {published data only}
-
- Boyle RG, Solberg LI, Asche SE, Boucher JL, Pronk NP, Jensen CJ. Offering telephone counseling to smokers using pharmacotherapy. Nicotine & Tobacco Research 2005;7 Suppl 1:S19‐27. - PubMed
-
- Boyle RG, Solberg LI, Asche SE, Maciosek MV, Boucher JL, Pronk NP. Proactive recruitment of health plan smokers into telephone counseling. Nicotine & Tobacco Research 2007;9(5):581‐9. - PubMed
Bricker 2014 {published data only}
-
- Heffner JL, Mull K, Mercer L, Vilardaga R, Bricker J. Effects of two telephone‐delivered smoking cessation interventions on hazardous drinking rates: ACT vs. CBT. Alcoholism, Clinical and Experimental Research 2014;38(Suppl 1):137A. [CENTRAL: 993969; CRS: 9400129000002292; EMBASE: 71503629]
Brody 2017 {published data only}
Brown 2013 {published data only}
-
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, et al. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine & Tobacco Research 2013;15(12):2005‐15. [CENTRAL: 915430; CRS: 9400129000000262; EMBASE: 2013716990; PUBMED: 23884317] - PMC - PubMed
Busch 2017 {published data only}
Bushnell 1997 {published data only}
-
- Bushnell FK, Forbes B, Goffaux J, Dietrich M, Wells N. Smoking cessation in military personnel. Military Medicine 1997;162(11):715‐9. - PubMed
Calabro 2012 {published data only}
-
- Calabro KS, Marani S, Yost T, Segura J, Mullin Jones M, Nelson S, et al. Project SUCCESS: results from a randomized controlled trial. International Scholarly Research Network Public Health 2012;2012:1‐9. [CENTRAL: 836719; CRS: 9400123000016091]
-
- Prokhorov AV, Marani S, Vost T, Luca M, Mullen M. Project SUCCESS: students using computerized coaching to end smoking successfully. Society for Research on Nicotine & Tobacco 17th Annual Meeting, 2011 February 16‐19; Toronto. 2011:18. [CENTRAL: 795274; CRS: 9400123000006112; 31113; PA2‐3]
Cook 2016 {published data only}
Cropsey 2015 {published data only}
Ellerbeck 2009 {published data only}
-
- Cupertino AP, Mahnken JD, Richter K, Cox LS, Casey G, Resnicow K, et al. Long‐term engagement in smoking cessation counseling among rural smokers. Journal of Health Care for the Poor and Underserved 2007;18(4 Suppl):30‐51. - PubMed
Ferguson 2012 {published data only}
Fiore 2004 {published data only}
-
- Fiore MC, McCarthy DE, Jackson TC, Zehner ME, Jorenby DE, Mielke M, et al. Integrating smoking cessation treatment into primary care: an effectiveness study. Preventive Medicine 2004;38(4):412‐20. - PubMed
Gariti 2009 {published data only}
Gifford 2011 {published data only}
-
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy 2011;42(4):700‐15. [CENTRAL: 814642; CRS: 9400123000012398; 6939; PUBMED: 22035998] - PubMed
Ginsberg 1992 {published data only}
-
- Ginsberg D, Hall SM, Rosinski M. Partner support, psychological treatment, and nicotine gum in smoking treatment: an incremental study. International Journal of the Addictions 1992;27(5):503‐14. - PubMed
Hall 1985 {published data only}
-
- Hall SM, Killen JD. Psychological and pharmacological approaches to smoking relapse prevention. National Institute on Drug Abuse Research Monograph 1985;53:131‐43. - PubMed
-
- Hall SM, Tunstall C, Rugg D, Jones R, Benowitz N. Nicotine gum and behavioral treatment in smoking cessation. Journal of Consulting and Clinical Psychology 1985;53(2):256‐8. - PubMed
Hall 1987 {published data only}
-
- Hall SM, Tunstall CD, Ginsberg D, Benowitz NL, Jones RT. Nicotine gum and behavioral treatment: a placebo controlled trial. Journal of Consulting and Clinical Psychology 1987;55(4):603‐5. - PubMed
Hall 1994 {published data only}
-
- Hall SM, Muñoz RF, Reus VI. Cognitive‐behavioral intervention increases abstinence rates for depressive‐history smokers. Journal of Consulting and Clinical Psychology 1994;62(1):141‐6. - PubMed
Hall 1998 {published data only}
-
- Hall SM, Reus VI, Muñoz RF, Sees KL, Humfleet G, Hartz DT, et al. Nortriptyline and cognitive‐behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry 1998;55(8):683‐90. - PubMed
-
- Hall SM, Reus VI, Muñoz RF, Sees KL, Humfleet GL, Frederick S. Nortriptyline and cognitive‐behavioral treatment of cigarette smoking. College on Problems of Drug Dependence Annual Meeting; 1996; San Juan, Puerto Rico. 1996:52.
Hall 2002 {published data only}
-
- Hall SM, Humfleet GL, Reus VI, Muñoz RF, Hartz DT, Maude‐Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry 2002;59(10):930‐6. - PubMed
-
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Muñoz R. Cost‐effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services & Research 2005;32(4):381‐92. - PubMed
Hall 2009 {published data only}
Hasan 2014 {published data only}
-
- Hasan FM, Zagarins SE, Pischke KM, Saiyed S, Bettencourt AM, Beal L, et al. Hypnotherapy is more effective than nicotine replacement therapy for smoking cessation: results of a randomized controlled trial. Complementary Therapies in Medicine 2014;22:1‐8. - PubMed
-
- NCT01791803. Smoking cessation after hospitalization for a cardiopulmonary illness (STOPP). ClinicalTrials.gov/show/NCT01791803 (first received 15 February 2013).
Hollis 2007 {published data only}
Huber 2003 {published data only}
-
- Huber D. Combined and separate treatment effects of nicotine chewing gum and self‐control method. Pharmacopsychiatry 1988;21(6):461‐2. - PubMed
-
- Huber D, Gastner J. Smoking cessation: a comparison of behavior therapy, nicotine replacement therapy and their combination. Verhaltenstherapie und Verhaltenshmedizin 2003;24:167‐85.
Humfleet 2013 {published data only}
-
- Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine & Tobacco Research 2013;15(8):1436‐45. [CENTRAL: 861196; CRS: 9400123000017501; EMBASE: 2013472501; OI:SOURCE:: NLM. PMC3715392 [Available on 08/01/14]; PUBMED: 23430708] - PMC - PubMed
Jorenby 1995 {published data only}
-
- Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, et al. Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995;274(17):1347‐52. - PubMed
Kahler 2015 {published data only}
Killen 2008 {published data only}
Kim 2015 {published data only}
LaChance 2015 {published data only}
Lando 1997 {published data only}
-
- Rolnick SJ, Klevan D, Cherney L, Lando HA. Nicotine replacement therapy in a group model HMO. Health Maintenance Organization Practice 1997;11(1):34‐7. - PubMed
Lifrak 1997 {published data only}
-
- Lifrak P, Gariti P, Alterman AI, McKay J, Volpicelli J, Sparkman T, et al. Results of two levels of adjunctive treatment used with the nicotine patch. American Journal on Addictions 1997;6(2):93‐8. - PubMed
Lloyd‐Richardson 2009 {published data only}
MacLeod 2003 {published data only}
-
- Macleod ZR, Charles MA, Arnaldi VC, Adams IM. Telephone counselling as an adjunct to nicotine patches in smoking cessation: a randomised controlled trial. Medical Journal of Australia 2003;179(7):349‐52. - PubMed
Macpherson 2010a {published data only}
Matthews 2018 {published data only}
-
- Matthews AK, Steffen A, Kuhns L, Ruiz R, Ross N, Burke L, et al. Evaluation of a randomized clinical trial comparing the effectiveness of a culturally targeted and non‐targeted smoking cessation intervention for lesbian, gay, bisexual and transgender (LGBT) smokers. Nicotine & Tobacco Research 2018;31:nty184. - PMC - PubMed
-
- NCT01633567. Culturally targeted & individually tailored smoking cessation study: LGBT smokers. ClinicalTrials.gov/show/NCT01633567 (first received 4 July 2012).
McCarthy 2008 {published data only}
-
- McCarthy DE. Mechanisms of tobacco cessation treatment: self‐report mediators of counseling and bupropion sustained release treatment. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67(9‐B):5414.
-
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research 2008;10(4):717‐29. - PubMed
NCT00879177 {published data only}
-
- NCT00879177. Smoking study with behavioral therapy for hypertensive patients. ClinicalTrials.gov/show/NCT00879177 (first received 9 April 2009).
O'Cleirigh 2018 {published data only}
-
- NCT01393301. Integrated treatment for smoking cessation & anxiety in people with HIV. ClinicalTrials.gov/show/NCT01393301 (first received 13 July 2011).
Ockene 1991 {published data only}
-
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician‐delivered smoking interventions: a randomized clinical trial. Journal of General Internal Medicine 1991;6(1):1‐8. - PubMed
-
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician‐delivered smoking intervention project: can short‐term interventions produce long‐term effects for a general outpatient population?. Health Psychology 1994;13(3):278‐81. - PubMed
Okuyemi 2013 {published data only}
-
- Goldade K, Jarlais D, Everson‐Rose SA, Guo H, Thomas J, Gelberg L, et al. Knowing quitters predicts smoking cessation in a homeless population. American Journal of Health Behavior 2013;37(4):517‐24. [CENTRAL: 993944; CRS: 9400107000000703; PEER: Reviewed Journal: 2013‐08953‐009; PUBMED: 23985232] - PMC - PubMed
-
- Goldade K, Whembolua GL, Thomas J, Eischen S, Guo H, Connett J, et al. Designing a smoking cessation intervention for the unique needs of homeless persons: a community‐based randomized clinical trial. Clinical Trials 2011;8(6):744‐54. [CENTRAL: 814647; CRS: 9400123000012422; 6866; PUBMED: 22167112] - PMC - PubMed
-
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Guo H, et al. Smoking characteristics and comorbidities in the power to quit randomized clinical trial for homeless smokers. Nicotine & Tobacco Research 2013;15(1):22‐8. [CENTRAL: 814589; CRS: 9400103000000015; EMBASE: 2012756123; PUBMED: 22589422] - PMC - PubMed
-
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Sewali B, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 2013;108(6):1136‐44. [CENTRAL: 921008; CRS: 9400107000001568; PEER: Reviewed Journal: 2013‐16822‐021; PUBMED: 23510102] - PMC - PubMed
Otero 2006 {published data only}
-
- Otero UB, Perez CA, Szklo M, Esteves GA, DePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive‐behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Ensaio clinico randomizado: efetividade da abordagem cognitivo‐comportamental e uso de adesivos transdermicos de reposicao de nicotina, na cessacao de fumar, em adultos residentes no Municipio do Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2006;22:439‐49. - PubMed
Patten 2017 {published data only}
Prapavessis 2016 {published data only}
-
- Prapavessis H, Jesus S, Fitzgeorge L, Faulkner G, Maddison R, Batten S. Exercise to enhance smoking cessation: the getting physical on cigarette randomized control trial. Annals of Behavioral Medicine 2016;50(3):358‐69. - PubMed
Reid 1999 {published data only}
Rohsenow 2014 {published data only}
-
- Rohsenow DJ, Martin RA, Monti PM, Colby SM, Day AM, Abrams DB, et al. Motivational interviewing versus brief advice for cigarette smokers in residential alcohol treatment. Journal of Substance Abuse Treatment 2014;46(3):346‐55. [CENTRAL: 959232; CRS: 9400130000000480; EMBASE: 2014038589; PUBMED: 24210533] - PMC - PubMed
-
- Rohsenow DJ, Monti PM, Colby SM, Martin RA. Brief interventions for smoking cessation in alcoholic smokers. Alcoholism, Clinical and Experimental Research 2002;26(12):1950‐1. [CENTRAL: 434257; CRS: 9400123000003065; PUBMED: 12500132] - PubMed
Rovina 2009 {published data only}
-
- Rovina N, Nikoloutsou I, Katsani G, Dima E, Fransis K, Roussos C, et al. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Therapeutic Advances in Respiratory Disease 2009;3(6):279‐87. - PubMed
Schlam 2016 {published data only}
Schmitz 2007a {published data only}
-
- Schmitz JM, Stotts AL, Mooney ME, Delaune KA, Moeller GF. Bupropion and cognitive‐behavioral therapy for smoking cessation in women. Nicotine & Tobacco Research 2007;9(6):699‐709. - PubMed
Simon 2003 {published data only}
-
- Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: a randomized trial. American Journal of Medicine 2003;114(7):555‐62. - PubMed
Smith 2001 {published data only}
-
- Smith SS, Jorenby DE, Fiore MC, Anderson JE, Mielke MM, Beach KE, et al. Strike while the iron is hot: can stepped‐care treatments resurrect relapsing smokers?. Journal of Consulting and Clinical Psychology 2001;69(3):429‐39. - PubMed
Smith 2013a {published data only}
Smith 2014 {published data only}
-
- NCT01083654. Menominee stop tobacco abuse renew tradition study. ClinicalTrials.gov/show/NCT01083654 (first received 10 March 2010).
Solomon 2000 {published data only}
-
- Solomon LJ, Scharoun GM, Flynn BS, Secker‐Walker RH, Sepinwall D. Free nicotine patches plus proactive telephone peer support to help low‐income women stop smoking. Preventive Medicine 2000;31(1):68‐74. - PubMed
Solomon 2005 {published data only}
-
- Solomon LJ, Marcy TW, Howe KD, Skelly JM, Reinier K, Flynn BS. Does extended proactive telephone support increase smoking cessation among low‐income women using nicotine patches?. Preventive Medicine 2005;40(3):306‐13. - PubMed
Stanton 2015 {published data only}
Stein 2006 {published data only}
-
- Stein MD, Anderson BJ, Niaura R. Smoking cessation patterns in methadone‐maintained smokers. Nicotine & Tobacco Research 2007;9(3):421‐8. - PubMed
-
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone‐maintained. Addiction 2006;101(4):599‐607. - PubMed
Strong 2009 {published data only}
-
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd‐Richardson E, Niaura R, et al. Impact of bupropion and cognitive‐behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research 2009;11(10):1142‐53. - PMC - PubMed
Swan 2003 {published data only}
-
- Jack LM, Swan GE, Thompson E, Curry SJ, McAfee T, Dacey S, et al. Bupropion SR and smoking cessation in actual practice: methods for recruitment, screening, and exclusion for a field trial in a managed‐care setting. Preventive Medicine 2003;36(5):585‐93. - PubMed
-
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker DL, et al. Cost‐effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective. American Journal of Managed Care 2004;10(3):217‐26. - PubMed
-
- Swan GE, Jack LM, Curry S, Chorost M, Javitz H, McAfee T, et al. Bupropion SR and counseling for smoking cessation in actual practice: predictors of outcome. Nicotine & Tobacco Research 2003;5(6):911‐21. - PubMed
-
- Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12‐month outcome in smokers who received bupropion sustained‐release for smoking cessation. Central Nervous System Drugs 2008;22(3):239‐56. - PubMed
-
- Swan GE, Javitz HS, Jack LM, Curry SJ, McAfee T. Heterogeneity in 12‐month outcome among female and male smokers. Addiction 2004;99(2):237‐50. - PubMed
Swan 2010 {published data only}
-
- McClure JB, Jack L, Deprey M, Catz S, McAfee T, Zbikowski S, et al. Canary in a coal mine? Interest in bupropion SR use among smokers in the COMPASS trial. Nicotine & Tobacco Research 2008;10(12):1815‐6. - PubMed
Tonnesen 2006 {published data only}
-
- Tonnesen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130(2):334‐42. - PubMed
Vander Weg 2016 {published data only}
-
- NCT01592695. Tailored tobacco quitline for rural veterans. ClinicalTrials.gov/show/NCT01592695 (first received 7 March 2012).
Van Rossem 2017 {published data only}
-
- Rossem C, Spigt M, Smit ES, Viechtbauer W, Mijnheer KK, Schayck CP, et al. Combining intensive practice nurse counselling or brief general practitioner advice with varenicline for smoking cessation in primary care: study protocol of a pragmatic randomized controlled trial. Contemporary Clinical Trials 2015;41:298‐312. [CENTRAL: 1066790; CRS: 9400131000001477; EMBASE: 2015828707] - PubMed
-
- Rossem C, Spigt M, Viechtbauer W, Lucas AEM, Schayck OCP, Kotz D. Effectiveness of intensive practice nurse counselling versus brief general practitioner advice, both combined with varenicline, for smoking cessation: a randomized pragmatic trial in primary care. Addiction 2017;112(12):2237‐47. - PubMed
Vidrine 2016 {published data only}
-
- Vidrine JI, Businelle MS, Reitzel LR, Cao Y, Cinciripini PM, Marcus MT, et al. Coping mediates the association of mindfulness with psychological stress, affect, and depression among smokers preparing to quit. Mindfulness 2014 [Epub ahead of print]. [CENTRAL: 1038659; CRS: 9400129000001024] - PMC - PubMed
Wagner 2016 {published data only}
Warner 2016 {published data only}
-
- Warner DO, Nolan MB, Kadimpati S, Burke MV, Hanson AC, Schroeder DR. Quitline tobacco interventions in hospitalized patients: a randomized trial. American Journal of Preventive Medicine 2016;51(4):473‐84. - PubMed
Webb Hooper 2017 {published data only}
-
- Webb Hooper M, Antoni MH, Okuyemi K, Dietz NA, Resnicow K. Randomized controlled trial of group‐based culturally specific cognitive behavioral therapy among African American smokers. Nicotine & Tobacco Research 2017;19(3):333‐41. - PubMed
Wewers 2017 {published data only}
Wiggers 2006 {published data only}
-
- Wiggers LC, Oort FJ, Dijkstra A, Haes JC, Legemate DA, Smets EM. Cognitive changes in cardiovascular patients following a tailored behavioral smoking cessation intervention. Preventive Medicine 2005;40(6):812‐21. - PubMed
-
- Wiggers LC, Oort FJ, Peters RJ, Legemate DA, Haes HC, Smets EM. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine & Tobacco Research 2006;8(4):581‐9. - PubMed
-
- Wiggers LC, Smets EM, Oort FJ, Peters RJ, Storm‐Versloot MN, Vermeulen H, et al. The effect of a minimal intervention strategy in addition to nicotine replacement therapy to support smoking cessation in cardiovascular outpatients: a randomized clinical trial. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(6):931‐7. - PubMed
-
- Wiggers LC, Smets EM, Oort FJ, Storm‐Versloot MN, Vermeulen H, Loenen LB, et al. Adherence to nicotine replacement patch therapy in cardiovascular patients. International Journal of Behavioral Medicine 2006;13(1):79‐88. - PubMed
Williams 2010 {published data only}
Wu 2009 {published data only}
Yalcin 2014 {published data only}
-
- Yalcin BM, Unal M, Pirdal H, Karahan TF. Effects of an anger management and stress control program on smoking cessation: a randomized controlled trial. Journal of the American Board of Family Medicine 2014;27(5):645‐60. [CENTRAL: 1014527; CRS: 9400129000003402] - PubMed
References to studies excluded from this review
Asfar 2010 {unpublished data only}
-
- Asfar T, Klesges RC, Sanford SD, Sherrill‐Mittleman D, Robison LL, Hudson MM, et al. Trial design: the St Jude Children's Research Hospital Cancer Survivors Tobacco Quit Line study. Contemporary Clinical Trials 2010;31(1):82‐91. [CENTRAL: 742881; CRS: 9400123000005579; PUBMED: 19766734] - PMC - PubMed
Bastian 2013 {published data only}
-
- Bastian LA, Fish LJ, Peterson BL, Biddle AK, Garst J, Lyna P, et al. Assessment of the impact of adjunctive proactive telephone counseling to promote smoking cessation among lung cancer patients' social networks. American Journal of Health Promotion 2013;27(3):181‐90. [CENTRAL: 921003; CRS: 9400107000000800; PEER: Reviewed Journal: 2013‐01449‐008; PUBMED: 23286595] - PubMed
Batra 2010 {published data only}
-
- Batra A, Collins SE, Schröter M, Eck S, Torchalla I, Buchkremer G, et al. A cluster‐randomized effectiveness trial of smoking cessation modified for at‐risk smoker subgroups. Journal of Substance Abuse Treatment 2010;38(2):128‐40. - PubMed
Bock 2008 {published data only}
-
- Bock BC, Becker BM, Niaura RS, Partridge R, Fava JL, Trask P. Smoking cessation among patients in an emergency chest pain observation unit: outcomes of the Chest Pain Smoking Study (CPSS). Nicotine & Tobacco Research 2008;10(10):1523‐31. - PubMed
Bonevski 2018 {published data only}
-
- Bonevski B, Twyman L, Paul C, D'Este C, West R, Siahpush M, et al. Smoking cessation intervention delivered by social service organisations for a diverse population of Australian disadvantaged smokers: a pragmatic randomised controlled trial. Preventive Medicine 2018;112:38‐44. - PubMed
Borland 2008 {published data only}
-
- Borland R, Balmford J, Bishop N, Segan C, Piterman L, McKay‐Brown L, et al. In‐practice management versus quitline referral for enhancing smoking cessation in general practice: a cluster randomized trial. Family Practice 2008;25(5):382‐9. - PubMed
-
- McKay‐Brown L, Borland R, Balmford J, Segan CJ, Andrews C, Tasker C, et al. The challenges of recruiting and retaining GPs in research: findings from a smoking cessation project. Australian Journal of Primary Health 2007;13(1):61‐7.
Brandon 2017 {published data only}
Breland 2014 {published data only}
-
- Breland AB, Almond L, Kienzle J, Ondersma SJ, Hart AJ, Weaver M, et al. Targeting tobacco in a community‐based addiction recovery cohort: results from a computerized, brief, randomized intervention trial. Contemporary Clinical Trials 2014;38(1):113‐20. [CENTRAL: 994445; EMBASE: 2014317617; PUBMED: 24721481] - PubMed
Brown 2007 {published data only}
-
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd‐Richardson E, Niaura R, et al. Impact of bupropion and cognitive‐behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research 2009;11(10):1142‐53. - PMC - PubMed
Buchanan 2004 {published data only}
-
- Buchanan LM, Banna M, White A, Moses S, Siedlik C, Wood M. An exploratory study of multicomponent treatment intervention for tobacco dependency. Journal of Nursing Scholarship 2004;36(4):324‐30. - PubMed
Carlin‐Menter 2011 {published data only}
-
- Carlin‐Menter S, Cummings KM, Celestino P, Hyland A, Mahoney MC, Willett J, et al. Does offering more support calls to smokers influence quit success?. Journal of Public Health Management and Practice 2011;17(3):E9‐15. - PubMed
Chandrashekar 2015 {published data only}
-
- Chandrashekar M, Sattar FA, Bondade S, Kiran Kumar K. A comparative study of different modalities of treatment in nicotine dependence syndrome. Asian Journal of Psychiatry 2015;17:29‐35. - PubMed
Chouinard 2005 {published data only}
-
- Chouinard MC, Robichaud‐Ekstrand S. The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease. Nursing Research 2005;54(4):243‐54. - PubMed
Christenhusz 2007 {published data only}
-
- Christenhusz L, Pieterse M, Seydel E, Palen J. Prospective determinants of smoking cessation in COPD patients within a high intensity or a brief counseling intervention. Patient Education & Counseling 2007;66(2):162‐6. - PubMed
Cooney 2007 {published data only}
-
- Cooney N, Pilkey D, Steinberg H, Cooney J, Litt M, Oncken C. Ecological momentary assessment of alcohol‐tobacco interactions after concurrent alcohol‐tobacco treatment. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
-
- Cooney N, Pilkey D, Steinberg H, Cooney J, Litt M, Oncken C. Efficacy of intensive versus brief smoking cessation treatment concurrent with intensive outpatient alcohol treatment. Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003 February 19‐22; New Orleans, Louisiana. 2003. [POS4‐62]
Cooper 2004 {published data only}
-
- Cooper TV, Debon MW, Stockton M, Klesges RC, Steenbergh TA, Sherrill‐Mittleman D, et al. Correlates of adherence with transdermal nicotine. Addictive Behaviors 2004;29(8):1565‐78. - PubMed
Costello 2011 {published data only}
-
- Costello MJ, Sproule B, Victor JC, Leatherdale ST, Zawertailo L, Selby P. Effectiveness of pharmacist counseling combined with nicotine replacement therapy: a pragmatic randomized trial with 6,987 smokers. Cancer Causes and Control 2011;22(2):167‐80. - PubMed
Cropsey 2017 {published data only}
Cummins 2016 {published data only}
Dezee 2013 {published data only}
-
- Dezee KJ, Wink JS, Cowan CM. Internet versus in‐person counseling for patients taking varenicline for smoking cessation. Military Medicine 2013;178(4):401‐5. [CENTRAL: 959429; CRS: 9400130000000072; PUBMED: 23707824] - PubMed
Emmons 2013 {published data only}
-
- Moor JS, Puleo E, Ford JS, Greenberg M, Hodgson DC, Tyc VL, et al. Disseminating a smoking cessation intervention to childhood and young adult cancer survivors: baseline characteristics and study design of the partnership for health‐2 study. BMC Cancer 2011;11:165. [CENTRAL: 836717; CRS: 9400123000011658; PUBMED: 21569345] - PMC - PubMed
-
- Emmons KM, Puleo E, Sprunck‐Harrild K, Ford J, Ostroff JS, Hodgson D, et al. Partnership for health‐2, a web‐based versus print smoking cessation intervention for childhood and young adult cancer survivors: randomized comparative effectiveness study. Journal of Medical Internet Research 2013;15(11):e218. [CENTRAL: 993946; CRS: 9400126000000377; PUBMED: 24195867] - PMC - PubMed
Evins 2007 {published data only}
-
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12‐week double‐blind, placebo‐controlled study of bupropion SR added to high‐dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Journal of Clinical Psychopharmacology 2007;27:380‐6. - PubMed
-
- NCT00320723. Nicotine replacement therapy for smoking cessation in schizophrenia. ClinicalTrials.gov/show/NCT00320723 (first received 3 May 2006).
Fang 2006 {published data only}
-
- Fang CY, Ma GX, Miller SM, Tan Y, Su X, Shive S. A brief smoking cessation intervention for Chinese and Korean American smokers. Preventive Medicine 2006;43(4):321‐4. - PubMed
Garvey 2012a {published data only}
-
- Garvey AJ, Hoskinson RA, Wadler BM, Wood EE, Kinnunen T, Kalman D, et al. Effects of front‐loaded vs. standard weekly counseling on 6‐month abstinence rates. Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21‐24; Austin, Texas. 2007. [POS1‐66]
Hall 1996 {published data only}
-
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment ‐ a therapeutic contact and placebo‐controlled study. Journal of Consulting and Clinical Psychology 1996;64(5):1003‐9. - PubMed
Hall 2004 {published data only}
-
- Hall SM, Humfleet GL, Reus VI, Muñoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry 2004;161(11):2100‐7. - PubMed
Hall 2011 {published data only}
Hegaard 2003 {published data only}
-
- Hegaard HK, Kjaergaard H, Møller LF, Wachmann H, Ottesen B. Multimodal intervention raises smoking cessation rate during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2003;82(9):813‐9. - PubMed
Ingersoll 2009 {published data only}
Japuntich 2006 {published data only}
-
- Japuntich SJ, Zehner ME, Smith SS, Jorenby DE, Valdez JA, Fiore MC, et al. Smoking cessation via the internet: a randomized clinical trial of an internet intervention as adjuvant treatment in a smoking cessation intervention. Nicotine & Tobacco Research 2006;8 Suppl 1:S59‐67. - PubMed
Joseph 2004 {published data only}
-
- Joseph AM, Nelson DB, Nugent SM, Willenbring ML. Timing of alcohol and smoking cessation (TASC): smoking among substance use patients screened and enrolled in a clinical trial. Journal of Addictive Disease 2003;22(4):87‐107. - PubMed
-
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. Journal of Studies on Alcohol 2004;65(6):681‐91. - PubMed
Joyce 2008 {published data only}
Kim 2012 {published data only}
Kinnunen 2008 {published data only}
Klesges 2015 {published data only}
-
- Berger VW. Randomization, permuted blocks, masking, allocation concealment, and selection bias in the Tobacco Quit Line study. Contemporary Clinical Trials 2010;31(3):201. [CENTRAL: 863870; CRS: 9400123000018617] - PubMed
Kotz 2009 {published data only}
-
- Kotz D, Wesseling G, Huibers MJ, Kotz D, Wesseling G, Huibers MJ, et al. Efficacy of confronting smokers with airflow limitation for smoking cessation. European Respiratory Journal 2009;33:754‐62. - PubMed
Levine 2010 {published data only}
Marshall 1985 {published data only}
McCarthy 2016 {published data only}
Moadel 2012 {published data only}
-
- Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV‐infected smokers. Journal of Acquired Immune Deficiency Syndromes 2012;61(2):208‐15. [CENTRAL: 839925; CRS: 9400123000016203; EMBASE: 2012577962; PEER: Reviewed Journal: 2012‐26825‐008; PUBMED: 22732470] - PMC - PubMed
Mochizuki 2004 {published data only}
-
- Mochizuki M, Hatsugaya M, Rokujoh E, Arita E, Hashiguchi M, Shimizu N, et al. Randomized controlled study on the effectiveness of community pharmacists' advice for smoking cessation by Nicorette ‐ evaluation at three months after initiation. Yakugaku Zasshi 2004;124(12):989‐95. - PubMed
NCT00781599 {published data only}
-
- NCT00781599. Improving medication compliance and smoking cessation treatment outcomes for African American smokers. ClinicalTrials.gov/show/NCT00781599 (first received 29 October 2008).
Nilsson 1996 {published data only}
-
- Nilsson P, Lundgren H, Söderström M, Fagerström KO, Nilsson Ehle P. Effects of smoking cessation on insulin and cardiovascular risk factors ‐ a controlled study of 4 months' duration. Journal of Internal Medicine 1996;240(4):189‐94. - PubMed
Nollen 2007 {published data only}
-
- Nollen N, Ahluwalia JS, Mayo MS, Richter K, Choi WS, Okuyemi KS, et al. A randomized trial of targeted educational materials for smoking cessation in African Americans using transdermal nicotine. Health Education & Behavior 2007;34(6):911‐27. [CENTRAL: 627383; CRS: 9400123000004636; PUBMED: 17576774] - PubMed
Nollen 2011 {published data only}
Okuyemi 2006 {published data only}
-
- Okuyemi KS, Thomas JL, Hall S, Nollen NL, Richter KP, Jeffries SK, et al. Smoking cessation in homeless populations: a pilot clinical trial. Nicotine & Tobacco Research 2006;8(5):689‐99. - PubMed
Pakhale 2015 {published data only}
Peckham 2015 {published data only}
-
- Peckham E, Man MS, Mitchell N, Li J, Becque T, Knowles S, et al. Smoking Cessation Intervention for severe Mental Ill Health Trial (SCIMITAR): a pilot randomised control trial of the clinical effectiveness and cost‐effectiveness of a bespoke smoking cessation service. Health Technology Assessment 2015;19(25):1‐148. [CRS: 9400131000001435; EMBASE: 2015892977; PUBMED: 25827850] - PMC - PubMed
Ramon 2013 {published data only}
-
- Ramon JM, Nerin I, Comino A, Pinet C, Abella F, Carreras JM, et al. A multicentre randomized trial of combined individual and telephone counselling for smoking cessation. Preventive Medicine 2013;57(3):183‐8. [CENTRAL: 871209; CRS: 9400107000001894; EMBASE: 2013515081; PUBMED: 23732247] - PubMed
Reid 2007 {published data only}
-
- Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: a pilot study. Patient Education & Counseling 2007;66(3):319‐26. - PubMed
Schnoll 2005 {published data only}
-
- Schnoll RA, Rothman RL, Wielt DB, Lerman C, Pedri H, Wang H, et al. A randomized pilot study of cognitive‐behavioral therapy versus basic health education for smoking cessation among cancer patients. Annals of Behavioral Medicine 2005;30(1):1‐11. - PubMed
Severson 2015 {published data only}
Shiffman 2000 {published data only}
-
- Shiffman S, Paty JA, Rohay JM, Marino ME, Gitchell J. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotinepolacrilex gum therapy. Archives of Internal Medicine 2000;160(11):1675‐81. - PubMed
Shiffman 2001 {published data only}
-
- Shiffman S, Paty JA, Rohay JM, Marino ME, Gitchell JG. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotine patch therapy. Drug & Alcohol Dependence 2001;64(1):35‐46. - PubMed
Sorensen 2003 {published data only}
-
- Sorensen LT, Jorgensen T. Short‐term preoperative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomised clinical trial. Colorectal Disease 2003;5:347‐52. - PubMed
Strecher 2005 {published data only}
-
- Strecher VJ, Shiffman S, West R. Moderators and mediators of a web‐based computer‐tailored smoking cessation program among nicotine patch users. Nicotine & Tobacco Research 2006;8 Suppl 1:S95‐101. - PubMed
-
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web‐based computer‐tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction 2005;100(5):682‐8. - PubMed
Velicer 2006 {published data only}
-
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage‐based therapies in a population‐based effectiveness trial. Journal of Consulting and Clinical Psychology 2006;74(6):1162‐72. - PubMed
Vial 2002 {published data only}
-
- Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. Journal of Pharmacy Practice and Research 2002;32(1):57‐62.
Ward 2001 {published data only}
-
- Ward T. Using psychological insights to help people quit smoking. Journal of Advanced Nursing 2001;34(6):754‐9. - PubMed
Wilson 1988 {published data only}
-
- Wilson DM, Taylor DW, Gilbert JR, Best JA, Lindsay EA, Willms DG, et al. A randomized trial of a family physician intervention for smoking cessation. JAMA 1988;260(11):1570‐4. - PubMed
Wolfenden 2005 {published data only}
-
- Wolfenden L, Wiggers J, Knight J, Campbell E, Rissel C, Kerridge R, et al. A programme for reducing smoking in pre‐operative surgical patients: randomised controlled trial. Anaesthesia 2005;60(2):172‐9. - PubMed
Yu 2006 {published data only}
-
- Yu H, Zang Y, Lin J. The effect of the abstinence from smoking with nicotine replacement therapy combining with psychological and behavior intervention. Zhongguo Kangfu Yixue Zazhi 2006;21(12):1104‐6.
Zwar 2015 {published data only}
-
- Zwar NA, Richmond RL, Halcomb EJ, Furler JS, Smith JP, Hermiz O, et al. Quit in general practice: a cluster randomized trial of enhanced in‐practice support for smoking cessation. Family Practice 2015;32(2):173‐80. [CRS: 9400131000001410; EMBASE: 2015912086; PUBMED: 25670206] - PubMed
References to ongoing studies
ACTRN12614000876695 {published data only}
-
- ACTRN12614000876695. Improving radiotherapy outcomes with smoking cessation: feasibility trial in head and neck cancer patients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366605 (first received 7 July 2014).
Asfar 2018 {published data only}
-
- Asfar T, Caban‐Martinez AJ, McClure LA, Ruano‐Herreria EC, Sierra D, Gilford Clark G Jr, et al. A cluster randomized pilot trial of a tailored worksite smoking cessation intervention targeting Hispanic/Latino construction workers: intervention development and research design. Contemporary Clinical Trials 2018;67:47‐55. - PMC - PubMed
Choi 2011 {published data only}
Cummins 2012 {published data only}
-
- Cummins S, Zhu SH, Gamst A, Kirby C, Brandstein K, Klonoff‐Cohen H, et al. Nicotine patches and quitline counseling to help hospitalized smokers stay quit: study protocol for a randomized controlled trial. Trials 2012;13(1):128. [CENTRAL: 853500; CRS: 9400123000015205; EMBASE: 2012559958; PUBMED: 22853197] - PMC - PubMed
Garvey 2012b {published data only}
-
- Garvey AJ, Kalman D, Hoskinson RA, Kinnunen T, Armour CD, Copp S, et al. Effects of extended‐duration counseling vs. shorter‐duration counseling after 1.5 years of follow‐up (POS4‐49). Society for Research on Nicotine and Tobacco 18th Annual Meeting; 2012 March 13‐16; Houston, Texas. 2012:139. [CENTRAL: 814595; CRS: 9400103000000026]
Kim 2017 {published data only}
-
- Kim SS, Darwish S, Lee SA, Demarco RF. A pilot study of a smoking cessation intervention for women living with HIV: study protocol. Open Access Journal of Clinical Trials 2017;9:11‐20.
NCT00851357 {published data only}
-
- NCT00851357. Telephone counseling and the distribution of nicotine patches to smokers. Clinicaltrials.gov/ct2/show/NCT00851357 (first received 25 February 2009).
NCT00937235 {published data only}
-
- NCT00937235. Treatment of smoking among individuals with PTSD: a phase II, randomized study of varenicline and cognitive behavioral therapy. clinicaltrials.gov/show/NCT00937235 (first received 10 July 2009). [CRS: 9400131000001780]
NCT00984724 {published data only}
-
- NCT00984724. Reducing tobacco related health disparities. ClinicalTrials.gov/show/NCT00984724 (first received 25 September 2009).
NCT01063972 {published data only}
-
- NCT01063972. Smoking cessation in rural hospitals. ClinicalTrials.gov/show/NCT01063972 (first received 5 February 2010).
NCT01098955 {published data only}
-
- NCT01098955. Smoking cessation treatment for head & neck cancer patients: acceptance and commitment therapy. clinicaltrials.gov/show/NCT01098955 (first received 5 April 2010). [CRS: 9400131000001782]
NCT01162239 {published data only}
-
- NCT01162239. Maintaining nonsmoking. clinicaltrials.gov/show/NCT01162239 (first received 14 July 2010). [CRS: 9400131000001784]
NCT01186016 {published data only}
-
- NCT01186016. Developing genetic education for smoking cessation. ClinicalTrials.gov/show/NCT01186016 (first received 20 August 2010).
NCT01257490 {published data only}
-
- NCT01257490. Integrated smoking cessation treatment for low income community corrections. clinicaltrials.gov/show/NCT01257490 (first received 9 December 2010). [CRS: 9400107000001341]
NCT01736085 {published data only}
-
- NCT01736085. Providing free nicotine patches to quitline smokers. ClinicalTrials.gov/show/NCT01736085 (first received 29 November 2012).
NCT01800019 {published data only}
-
- NCT01800019. The Canadian HIV quit smoking trial: tackling the co‐morbidities of depression and cardiovascular disease in HIV+ smokers. clinicaltrials.gov/show/NCT01800019 (first received 27 February 2013). [CRS: 9400129000001211]
NCT01901848 {published data only}
-
- NCT01901848. CPT and smoking cessation. ClinicalTrials.gov/show/NCT01901848 (first received 17 July 2013).
NCT01965405 {published data only}
-
- NCT01965405. Smoking cessation for people living with HIV/AIDS. ClinicalTrials.gov/show/NCT01965405 (first received 18 October 2013).
NCT02048917 {published data only}
-
- NCT02048917. Optimization of smoking cessation strategies in community cancer programs for newly diagnosed lung and head and neck cancer patients. Clinicaltrials.gov/show/NCT02048917 (first received 29 January 2014). [CENTRAL: 1013511; CRS: 9400050000000094]
NCT02164383 {published data only}
-
- NCT02164383. A quit smoking study using smartphones. clinicaltrials.gov/show/NCT02164383 (first received 16 June 2014). [CRS: 9400131000001038]
NCT02378714 {published data only}
-
- NCT02378714. Behavioral activation and varenicline for smoking cessation in depressed smokers. Clinicaltrials.gov/ct2/show/NCT02378714 (first received 4 March 2015).
NCT02460900 {published data only}
-
- NCT02460900. Optimizing smoking cessation for people with HIV/AIDS who smoke. ClinicalTrials.gov/show/NCT02460900 (first recived 3 June 2015).
NCT02767908 {published data only}
-
- NCT02767908. Hospital to home, smoker support trial. ClinicalTrials.gov/show/NCT02767908 (first received 11 May 2016).
NCT02898597 {published data only}
-
- NCT02898597. Smoking cessation intervention for women living with HIV. ClinicalTrials.gov/show/NCT02898597 (first received 13 September 2016).
NCT02905656 {published data only}
-
- NCT02905656. Strategies to promote cessation in smokers who are not ready to quit. ClinicalTrials.gov/show/NCT02905656 (first received 19 September 2016).
NCT03072511 {published data only}
-
- NCT03072511. Smoking cessation intervention. ClinicalTrials.gov/show/NCT03072511 (first received 7 March 2017).
NCT03342027 {published data only}
-
- NCT03342027. Smoking cessation interventions for people living with HIV in Nairobi, Kenya. ClinicalTrials.gov/show/NCT03342027 (first received 14 November 2017).
NCT03538938 {published data only}
-
- NCT03538938. Improving quitline support study. ClinicalTrials.gov/show/NCT03538938 (first received 28 May 2018).
NCT03603496 {published data only}
-
- NCT03603496. Post‐discharge smoking cessation strategies: helping HAND 4. ClinicalTrials.gov/show/NCT03603496 (first received 27 July 2018).
Ojo‐Fati 2015 {published data only}
Powers 2016 {published data only}
-
- Powers MB, Kauffman BY, Kleinsasser AL, Lee‐Furman E, Smits JA, Zvolensky MJ, et al. Efficacy of smoking cessation therapy alone or integrated with prolonged exposure therapy for smokers with PTSD: study protocol for a randomized controlled trial. Contemporary Clinical Trials 2016;50:213‐21. - PMC - PubMed
Reid 2011 {published data only}
-
- Reid RD, Aitken DA, McDonnell L, Armstrong A, Mullen KA, Jones L, et al. Randomized trial of an automated telephone follow‐up system for smoking cessation in smokers with CHD. Canadian Journal of Cardiology 2011;27(5 Suppl 1):S67. [CENTRAL: 814379; CRS: 9400123000012493]
Salgado 2018 {published data only}
Vander Weg 2018 {published data only}
-
- Vander Weg MW, Coday M, Stockton MB, McClanahan B, Relyea G, Read MC, et al. Community‐based physical activity as adjunctive smoking cessation treatment: rationale, design, and baseline data for the Lifestyle Enhancement Program (LEAP) randomized controlled trial. Contemporary Clinical Trials Communications 2018;9:50‐9. - PMC - PubMed
Vidrine 2012 {published data only}
Webb 2018 {published data only}
-
- Webb Hooper M, Lee DJ, Simmons VN, Brandon KO, Antoni MH, Unrod M, et al. Reducing racial/ethnic tobacco cessation disparities via cognitive behavioral therapy: design of a dualsite randomized controlled trial. Contemporary Clinical Trials 2018;68:127‐32. - PubMed
Additional references
Cahill 2016
Cochrane Handbook 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.1 [Updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Cofta‐Woerpel 2007
-
- Cofta‐Woerpel L, Wright KL, Wetter DW. Smoking cessation 3: multicomponent interventions. Behavioral Medicine 2007;32(4):135‐49. - PubMed
Coleman 2015
Fiore 2008
-
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. AHRQ publication No. 00‐0032. Rockville, MD: US Dept of Health and Human Services, Public Health Services, 2008.
Hartmann‐Boyce 2018
Hartmann‐Boyce 2018a
Higgins 2003
Hughes 1995
-
- Hughes JR. Combining behavioral therapy and pharmacotherapy for smoking cessation: an update. National Institute on Drug Abuse Research Monograph 1995;150:92‐109. - PubMed
Hughes 2010
Hughes 2014
Lancaster 2017
Lindson‐Hawley 2015
Livingstone‐Banks 2019
Livingstone‐Banks 2019a
Matkin 2019
R program [Computer program]
-
- R Foundation for Statistical Computing. R. Version 3.5.2. R Foundation for Statistical Computing, 2018.
Rice 2017
Rigotti 2012
Stead 2016
Stead 2017
West 2007
-
- West R. The clinical significance of "small" effects of smoking cessation treatments. Addiction 2007;102(4):506‐9. - PubMed
West 2010
-
- West R, Walia A, Hyder N, Shahab L, Michie S. Behavior change techniques used by the English Stop Smoking Services and their associations with short‐term quit outcomes. Nicotine & Tobacco Research 2010;12(7):742‐7. - PubMed
Zhu 2000
-
- Zhu SH, Tedeschi GJ, Anderson CM, Rosbrook B, Byrd M, Johnson CE, et al. Telephone counseling as adjuvant treatment for nicotine replacement therapy in a "Real‐World" setting. Preventive Medicine 2000;31(4):357‐63. - PubMed
References to other published versions of this review
Stead 2012a
Stead 2012b
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

