Expression and distribution of PIEZO1 in the mouse urinary tract
- PMID: 31166705
- PMCID: PMC6732449
- DOI: 10.1152/ajprenal.00214.2019
Expression and distribution of PIEZO1 in the mouse urinary tract
Abstract
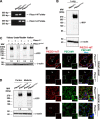
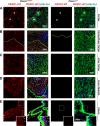
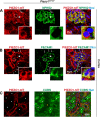
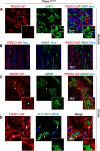
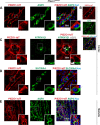
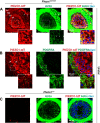
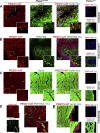
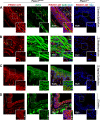
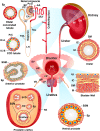
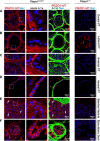
The proper function of the organs that make up the urinary tract (kidneys, ureters, bladder, and urethra) depends on their ability to sense and respond to mechanical forces, including shear stress and wall tension. However, we have limited understanding of the mechanosensors that function in these organs and the tissue sites in which these molecules are expressed. Possible candidates include stretch-activated PIEZO channels (PIEZO1 and PIEZO2), which have been implicated in mechanically regulated body functions including touch sensation, proprioception, lung inflation, and blood pressure regulation. Using reporter mice expressing a COOH-terminal fusion of Piezo1 with the sequence for the tandem-dimer Tomato gene, we found that PIEZO1 is expressed in the kidneys, ureters, bladder, and urethra as well as organs in close proximity, including the prostate, seminal vesicles and ducts, ejaculatory ducts, and the vagina. We further found that PIEZO1 expression is not limited to one cell type; it is observed in the endothelial and parietal cells of the renal corpuscle, the basolateral surfaces of many of the epithelial cells that line the urinary tract, the interstitial cells of the bladder and ureters, and populations of smooth and striated muscle cells. We propose that in the urinary tract, PIEZO1 likely functions as a mechanosensor that triggers responses to wall tension.
Keywords: PIEZO channel; bladder; kidney; mechanotransduction; urinary tract.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Fénéant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garçon L, Toutain F, Simon Rohrlich P, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun 4: 1884, 2013. [Erratum in Nat Commun 4: 2440, 2013.] doi: 10.1038/ncomms2899. - DOI - PMC - PubMed
-
- Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR, Vandorpe DH, Shmukler BE, Narayan R, Montanaro D, D’Armiento M, Vetro A, Limongelli I, Zuffardi O, Glader BE, Schrier SL, Brugnara C, Stewart GW, Delaunay J, Iolascon A. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121: 3925–3935, 2013. doi: 10.1182/blood-2013-02-482489. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

