A central core disease mutation in the Ca2+-binding site of skeletal muscle ryanodine receptor impairs single-channel regulation
- PMID: 31166712
- PMCID: PMC6732417
- DOI: 10.1152/ajpcell.00052.2019
A central core disease mutation in the Ca2+-binding site of skeletal muscle ryanodine receptor impairs single-channel regulation
Abstract
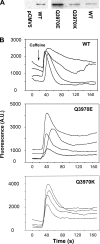
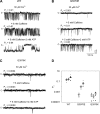
Cryoelectron microscopy and mutational analyses have shown that type 1 ryanodine receptor (RyR1) amino acid residues RyR1-E3893, -E3967, and -T5001 are critical for Ca2+-mediated activation of skeletal muscle Ca2+ release channel. De novo missense mutation RyR1-Q3970K in the secondary binding sphere of Ca2+ was reported in association with central core disease (CCD) in a 2-yr-old boy. Here, we characterized recombinant RyR1-Q3970K mutant by cellular Ca2+ release measurements, single-channel recordings, and computational methods. Caffeine-induced Ca2+ release studies indicated that RyR1-Q3970K formed caffeine-sensitive, Ca2+-conducting channel in HEK293 cells. However, in single-channel recordings, RyR1-Q3970K displayed low Ca2+-dependent channel activity and greatly reduced activation by caffeine or ATP. A RyR1-Q3970E mutant corresponds to missense mutation RyR2-Q3925E associated with arrhythmogenic syndrome in cardiac muscle. RyR1-Q3970E also formed caffeine-induced Ca2+ release in HEK293 cells and exhibited low activity in the presence of the activating ligand Ca2+ but, in contrast to RyR1-Q3970K, was activated by ATP and caffeine in single-channel recordings. Computational analyses suggested distinct structural rearrangements in the secondary binding sphere of Ca2+ of the two mutants, whereas the interaction of Ca2+ with directly interacting RyR1 amino acid residues Glu3893, Glu3967, and Thr5001 was only minimally affected. We conclude that RyR1-Q3970 has a critical role in Ca2+-dependent activation of RyR1 and that a missense RyR1-Q3970K mutant may give rise to myopathy in skeletal muscle.
Keywords: central core disease; homology modeling; ryanodine receptor; sarcoplasmic reticulum; single-channel recording.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Structural and functional interactions between the Ca2+-, ATP-, and caffeine-binding sites of skeletal muscle ryanodine receptor (RyR1).J Biol Chem. 2021 Sep;297(3):101040. doi: 10.1016/j.jbc.2021.101040. Epub 2021 Aug 2. J Biol Chem. 2021. PMID: 34352272 Free PMC article.
-
Ca2+-mediated activation of the skeletal-muscle ryanodine receptor ion channel.J Biol Chem. 2018 Dec 14;293(50):19501-19509. doi: 10.1074/jbc.RA118.004453. Epub 2018 Oct 19. J Biol Chem. 2018. PMID: 30341173 Free PMC article.
-
Channel Gating Dependence on Pore Lining Helix Glycine Residues in Skeletal Muscle Ryanodine Receptor.J Biol Chem. 2015 Jul 10;290(28):17535-45. doi: 10.1074/jbc.M115.659672. Epub 2015 May 21. J Biol Chem. 2015. PMID: 25998124 Free PMC article.
-
Ryanodine receptor mutations in malignant hyperthermia and central core disease.Hum Mutat. 2000;15(5):410-7. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. Hum Mutat. 2000. PMID: 10790202 Review.
-
Altered ryanodine receptor function in central core disease: leaky or uncoupled Ca(2+) release channels?Trends Cardiovasc Med. 2002 Jul;12(5):189-97. doi: 10.1016/s1050-1738(02)00163-9. Trends Cardiovasc Med. 2002. PMID: 12161072 Review.
Cited by
-
Ca2+ inactivation of the mammalian ryanodine receptor type 1 in a lipidic environment revealed by cryo-EM.Elife. 2022 Mar 8;11:e75568. doi: 10.7554/eLife.75568. Elife. 2022. PMID: 35257661 Free PMC article.
-
Point mutations in RyR2 Ca2+-binding residues of human cardiomyocytes cause cellular remodelling of cardiac excitation contraction-coupling.Cardiovasc Res. 2024 Feb 27;120(1):44-55. doi: 10.1093/cvr/cvad163. Cardiovasc Res. 2024. PMID: 37890099 Free PMC article.
-
Structural and functional interactions between the Ca2+-, ATP-, and caffeine-binding sites of skeletal muscle ryanodine receptor (RyR1).J Biol Chem. 2021 Sep;297(3):101040. doi: 10.1016/j.jbc.2021.101040. Epub 2021 Aug 2. J Biol Chem. 2021. PMID: 34352272 Free PMC article.
-
Understanding IP3R channels: From structural underpinnings to ligand-dependent conformational landscape.Cell Calcium. 2023 Sep;114:102770. doi: 10.1016/j.ceca.2023.102770. Epub 2023 Jun 22. Cell Calcium. 2023. PMID: 37393815 Free PMC article. Review.
-
Attempts to Create Transgenic Mice Carrying the Q3924E Mutation in RyR2 Ca2+ Binding Site.Cells. 2024 Dec 12;13(24):2051. doi: 10.3390/cells13242051. Cells. 2024. PMID: 39768143 Free PMC article.
References
-
- DeLano WL. The PyMOL Molecular Graphics System, version 1.5.0.4 New York: Schrödinger, 2011. https://pymol.org/2/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

