A central core disease mutation in the Ca2+-binding site of skeletal muscle ryanodine receptor impairs single-channel regulation
- PMID: 31166712
- PMCID: PMC6732417
- DOI: 10.1152/ajpcell.00052.2019
A central core disease mutation in the Ca2+-binding site of skeletal muscle ryanodine receptor impairs single-channel regulation
Abstract
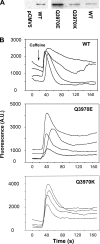
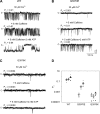
Cryoelectron microscopy and mutational analyses have shown that type 1 ryanodine receptor (RyR1) amino acid residues RyR1-E3893, -E3967, and -T5001 are critical for Ca2+-mediated activation of skeletal muscle Ca2+ release channel. De novo missense mutation RyR1-Q3970K in the secondary binding sphere of Ca2+ was reported in association with central core disease (CCD) in a 2-yr-old boy. Here, we characterized recombinant RyR1-Q3970K mutant by cellular Ca2+ release measurements, single-channel recordings, and computational methods. Caffeine-induced Ca2+ release studies indicated that RyR1-Q3970K formed caffeine-sensitive, Ca2+-conducting channel in HEK293 cells. However, in single-channel recordings, RyR1-Q3970K displayed low Ca2+-dependent channel activity and greatly reduced activation by caffeine or ATP. A RyR1-Q3970E mutant corresponds to missense mutation RyR2-Q3925E associated with arrhythmogenic syndrome in cardiac muscle. RyR1-Q3970E also formed caffeine-induced Ca2+ release in HEK293 cells and exhibited low activity in the presence of the activating ligand Ca2+ but, in contrast to RyR1-Q3970K, was activated by ATP and caffeine in single-channel recordings. Computational analyses suggested distinct structural rearrangements in the secondary binding sphere of Ca2+ of the two mutants, whereas the interaction of Ca2+ with directly interacting RyR1 amino acid residues Glu3893, Glu3967, and Thr5001 was only minimally affected. We conclude that RyR1-Q3970 has a critical role in Ca2+-dependent activation of RyR1 and that a missense RyR1-Q3970K mutant may give rise to myopathy in skeletal muscle.
Keywords: central core disease; homology modeling; ryanodine receptor; sarcoplasmic reticulum; single-channel recording.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- DeLano WL. The PyMOL Molecular Graphics System, version 1.5.0.4 New York: Schrödinger, 2011. https://pymol.org/2/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

