Defining consensus opinion to develop randomised controlled trials in rare diseases using Bayesian design: An example of a proposed trial of adalimumab versus pamidronate for children with CNO/CRMO
- PMID: 31166977
- PMCID: PMC6550371
- DOI: 10.1371/journal.pone.0215739
Defining consensus opinion to develop randomised controlled trials in rare diseases using Bayesian design: An example of a proposed trial of adalimumab versus pamidronate for children with CNO/CRMO
Abstract
Introduction: Chronic nonbacterial osteomyelitis (CNO) is a rare autoinflammatory bone disorder primarily affecting children and adolescents. It can lead to chronic pain, bony deformities and fractures. The pathophysiology of CNO is incompletely understood. Scientific evidence suggests dysregulated expression of pro- and anti-inflammatory cytokines to be centrally involved. Currently, treatment is largely based on retrospective observational studies and expert opinion. Treatment usually includes nonsteroidal anti-inflammatory drugs and/or glucocorticoids, followed by a range of drugs in unresponsive cases. While randomised clinical trials are lacking, retrospective and prospective non-controlled studies suggest effectiveness of TNF inhibitors and bisphosphonates. The objective of the Bayesian consensus meeting was to quantify prior expert opinion.
Methods: Twelve international CNO experts were randomly chosen to be invited to a Bayesian prior elicitation meeting.
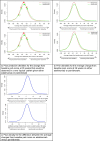
Results: Results showed that a typical new patient treated with pamidronate would have an 84% chance of improvement in their pain score relative to baseline at 26 weeks and an 83% chance on adalimumab. Experts thought there was a 50% chance that a new typical patient would record a pain score of 28mm (pamidronate) to 30mm (adalimumab) or better at 26 weeks. There was a modest trend in prior opinion to indicate an advantage of pamidronate vs adalimumab, with a 68% prior chance that pamidronate is superior to adalimumab by some margin. However, it is clear that there is considerable uncertainty about the precise relative merits of the two treatments.
Conclusions: The rarity of CNO leads to challenges in conducting randomised controlled trials with sufficient power to provide a definitive outcome. We address this using a Bayesian design, and here describe the process and outcome of the elicitation exercise to establish expert prior opinion. This opinion will be tested in the planned prospective CNO study. The process for establishing expert consensus opinion in CNO will be helpful for developing studies in other rare paediatric diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Brandt D, Sohr E, Pablik J, Schnabel A, Kapplusch F, Mäbert K, Girschick JH et al. CD14+ monocytes contribute to inflammation in chronic nonbacterial osteomyelitis (CNO) through increased NLRP3 inflammasome expression. Clin Immunol. 2018. April 30 pii: S1521–6616(18)30257-2. 10.1016/j.clim.2018.04.011 [Epub ahead of print] - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

