Longer-Term Assessment of Azithromycin for Reducing Childhood Mortality in Africa
- PMID: 31167050
- PMCID: PMC6512890
- DOI: 10.1056/NEJMoa1817213
Longer-Term Assessment of Azithromycin for Reducing Childhood Mortality in Africa
Abstract
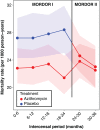
Background: The MORDOR I trial (Macrolides Oraux pour Réduire les Décès avec un Oeil sur la Résistance) showed that in Niger, mass administration of azithromycin twice a year for 2 years resulted in 18% lower postneonatal childhood mortality than administration of placebo. Whether this benefit could increase with each administration or wane owing to antibiotic resistance was unknown.
Methods: In the Niger component of the MORDOR I trial, we randomly assigned 594 communities to four twice-yearly distributions of either azithromycin or placebo to children 1 to 59 months of age. In MORDOR II, all these communities received two additional open-label azithromycin distributions. All-cause mortality was assessed twice yearly by census workers who were unaware of participants' original assignments.
Results: In the MORDOR II trial, the mean (±SD) azithromycin coverage was 91.3±7.2% in the communities that received twice-yearly azithromycin for the first time (i.e., had received placebo for 2 years in MORDOR I) and 92.0±6.6% in communities that received azithromycin for the third year (i.e., had received azithromycin for 2 years in MORDOR I). In MORDOR II, mortality was 24.0 per 1000 person-years (95% confidence interval [CI], 22.1 to 26.3) in communities that had originally received placebo in the first year and 23.3 per 1000 person-years (95% CI, 21.4 to 25.5) in those that had originally received azithromycin in the first year, with no significant difference between groups (P = 0.55). In communities that had originally received placebo, mortality decreased by 13.3% (95% CI, 5.8 to 20.2) when the communities received azithromycin (P = 0.007). In communities that had originally received azithromycin and continued receiving it for an additional year, the difference in mortality between the third year and the first 2 years was not significant (-3.6%; 95% CI, -12.3 to 4.5; P = 0.50).
Conclusions: We found no evidence that the effect of mass administration of azithromycin on childhood mortality in Niger waned in the third year of treatment. Childhood mortality decreased when communities that had originally received placebo received azithromycin. (Funded by the Bill and Melinda Gates Foundation; ClinicalTrials.gov number, NCT02047981.).
Copyright © 2019 Massachusetts Medical Society.
Figures

Comment in
-
Mass Administration of Azithromycin to Prevent Pre-school Childhood Mortality: Boon or Bane?: Evidence-based Medicine Viewpoint.Indian Pediatr. 2019 Sep 15;56(9):767-770. Indian Pediatr. 2019. PMID: 31638010 No abstract available.
-
Mass Administration of Azithromycin to Prevent Pre-school Childhood Mortality: Boon or Bane?: Pediatrician's Viewpoint.Indian Pediatr. 2019 Sep 15;56(9):770-771. Indian Pediatr. 2019. PMID: 31638011 No abstract available.
-
Macrolide Resistance and Longer-Term Assessment of Azithromycin in MORDOR I.N Engl J Med. 2019 Nov 28;381(22):2184. doi: 10.1056/NEJMc1910014. N Engl J Med. 2019. PMID: 31774978 No abstract available.
-
Mass administration of azithromycin reduces childhood mortality in Niger.Arch Dis Child Educ Pract Ed. 2021 Oct;106(5):320. doi: 10.1136/archdischild-2020-319718. Epub 2020 Sep 8. Arch Dis Child Educ Pract Ed. 2021. PMID: 32900778 No abstract available.
References
-
- O'Brien KS, Emerson P, Hooper PJ, et al. Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review. Lancet Infect Dis 2018. - PubMed
-
- Leach AJ, Shelby-James TM, Mayo M, et al. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clinical Infectious Diseases 1997;24:356-62. - PubMed
-
- Haug S, Lakew T, Habtemariam G, et al. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis 2010;51:571-4. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
