The Pleiotropic Effects of Glutamine Metabolism in Cancer
- PMID: 31167399
- PMCID: PMC6627534
- DOI: 10.3390/cancers11060770
The Pleiotropic Effects of Glutamine Metabolism in Cancer
Abstract
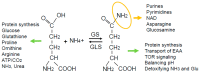
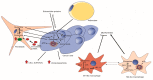
Metabolic programs are known to be altered in cancers arising from various tissues. Malignant transformation can alter signaling pathways related to metabolism and increase the demand for both energy and biomass for the proliferating cancerous cells. This scenario is further complexed by the crosstalk between transformed cells and the microenvironment. One of the most common metabolic alterations, which occurs in many tissues and in the context of multiple oncogenic drivers, is the increased demand for the amino acid glutamine. Many studies have attributed this increased demand for glutamine to the carbon backbone and its role in the tricarboxylic acid (TCA) cycle anaplerosis. However, an increasing number of studies are now emphasizing the importance of glutamine functioning as a proteogenic building block, a nitrogen donor and carrier, an exchanger for import of other amino acids, and a signaling molecule. Herein, we highlight the recent literature on glutamine's versatile role in cancer, with a focus on nitrogen metabolism, and therapeutic implications of glutamine metabolism in cancer.
Keywords: cancer; glutamate; glutamate ammonia ligase (GLUL); glutaminase (GLS); glutamine; glutamine synthetase (GS); glutaminolysis; metabolism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Commisso C., Davidson S.M., Soydaner-Azeloglu R.G., Parker S.J., Kamphorst J.J., Hackett S., Grabocka E., Nofal M., Drebin J.A., Thompson C.B., et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. - DOI - PMC - PubMed
-
- Griffiths B., Lewis C.A., Bensaad K., Ros S., Zhang Q., Ferber E.C., Konisti S., Peck B., Miess H., East P., et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab. 2013;1:3. doi: 10.1186/2049-3002-1-3. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

