Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics?
- PMID: 31167476
- PMCID: PMC6600223
- DOI: 10.3390/ijms20112747
Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics?
Abstract
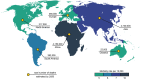
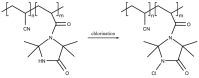
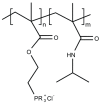
Antimicrobial resistance is now considered a major global challenge; compromising medical advancements and our ability to treat infectious disease. Increased antimicrobial resistance has resulted in increased morbidity and mortality due to infectious diseases worldwide. The lack of discovery of novel compounds from natural products or new classes of antimicrobials, encouraged us to recycle discontinued antimicrobials that were previously removed from routine use due to their toxicity, e.g., colistin. Since the discovery of new classes of compounds is extremely expensive and has very little success, one strategy to overcome this issue could be the application of synthetic compounds that possess antimicrobial activities. Polymers with innate antimicrobial properties or that have the ability to be conjugated with other antimicrobial compounds create the possibility for replacement of antimicrobials either for the direct application as medicine or implanted on medical devices to control infection. Here, we provide the latest update on research related to antimicrobial polymers in the context of ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) pathogens. We summarise polymer subgroups: compounds containing natural peptides, halogens, phosphor and sulfo derivatives and phenol and benzoic derivatives, organometalic polymers, metal nanoparticles incorporated into polymeric carriers, dendrimers and polymer-based guanidine. We intend to enhance understanding in the field and promote further work on the development of polymer based antimicrobial compounds.
Keywords: ESKAPE pathogens; antimicrobial polymers; antimicrobial resistance.
Conflict of interest statement
The views expressed by M.d.F.P are those of the author; they do not reflect in any way the views of the agency.
Figures
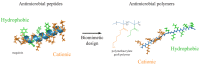


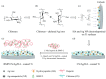
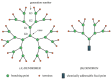
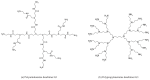
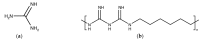
References
-
- WHO High Levels of Antibiotic Resistance Found Worldwide, New Data Shows. WHO, News Release. [(accessed on 29 January 2018)]; Available online: https://www.who.int/mediacentre/news/releases/2018/antibiotic-resistance...
-
- O’Neill J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014;1:1–16.
-
- Natarajan S.V., Dhanasekar U. Trends in antibiotic resistance pattern against ESKAPE pathogens from tertiary care teaching institute – South India. Infect. Dis. Heal. 2016;21:142–143. doi: 10.1016/j.idh.2016.09.118. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

