One-step Preparation of a VHH-based Immunoadsorbent for the Extracorporeal Removal of β2-microglobulin
- PMID: 31167508
- PMCID: PMC6600539
- DOI: 10.3390/molecules24112119
One-step Preparation of a VHH-based Immunoadsorbent for the Extracorporeal Removal of β2-microglobulin
Abstract
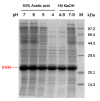
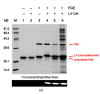
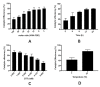
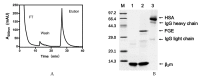
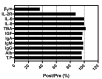
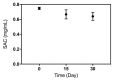
Dialysis-related amyloidosis (DRA), which has been widely recognized to be associated with the accumulation of β2-microglobulin (β2-m) in blood, is one of the most common complications in patients receiving long-term dialysis treatment. The most significant side-effect of existing hemodialysis sorbents for the removal of β2-m from blood is the loss of vital proteins due to non-specific adsorptions. Although the traditional antibodies have the capability to specifically remove β2-m from blood, high cost limits their applications in clinics. Single domain antibodies derived from the Camelidae species serve as a superior choice in the preparation of immunoadsorbents due to their small size, high stability, amenability, simplicity of expression in microbes, and high affinity to recognize and interact with β2-m. In this study, we modified the anti-β2-m VHH by the formylglycine-generating enzyme (FGE), and then directly immobilized the aldehyde-modified VHH to the amino-activated beads. Notably, the fabrication is cost- and time-effective, since all the preparation steps were performed in the crude cell extract without rigorous purification. The accordingly prepared immunoadsorbent with VHHs as ligands exhibited the high capacity of β2-m (0.75 mg/mL). In conclusion, the VHH antibodies were successfully used as affinity ligands in the preparation of novel immunoadsorbents by the site-specific immobilization, and effectively adsorbed β2-m from blood, therefore opening a new avenue for efficient hemodialysis.
Keywords: VHH; formylglycine-generating enzyme; hemodialysis; β2-microglobulin.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
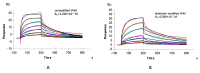


References
-
- Levey A.S., Eckardt K.U., Tsukamoto Y., Levin A., Coresh J., Rossert J., de Zeeuw D., Hostetter T.H., Lameire N., Eknoyan G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. - DOI - PubMed
-
- Levey A.S., Coresh J., Balk E., Kausz A.T., Levin A., Steffes M.W., Hogg R.J., Perrone R.D., Lau J., Eknoyan G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. - DOI - PubMed
-
- Eknoyan G., Levin N.W. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification-Foreword. Am. J. Kidney Dis. 2002;39:S14–S266. - PubMed
-
- Gejyo F., Homma N., Suzuki Y., Arakawa M. Serum Levels of β-2-Microglobulin as a New Form of Amyloid Protein in Patients Undergoing Long-Term Hemodialysis. New Engl. J. Med. 1986;314:585–586. - PubMed
-
- Ogawa H., Saito A., Hirabayashi N., Hara K. Amyloid Deposition in Systemic Organs in Long-Term Hemodialysis-Patients. Clin. Nephrol. 1987;28:199–204. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

