Simvastatin Attenuates Cardiac Fibrosis via Regulation of Cardiomyocyte-Derived Exosome Secretion
- PMID: 31167519
- PMCID: PMC6617127
- DOI: 10.3390/jcm8060794
Simvastatin Attenuates Cardiac Fibrosis via Regulation of Cardiomyocyte-Derived Exosome Secretion
Abstract
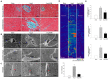
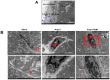
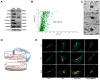
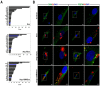
Exosome-mediated communication within the cardiac microenvironment is associated with cardiac fibrosis. Simvastatin (SIM), a potent statin, protects against cardiac fibrosis, but its mechanism of action is unclear. We investigated the inhibitory effects and underlying mechanism of simvastatin in cardiac fibrosis, by regulating exosome-mediated communication. Male Sprague-Dawley rats were treated with angiotensin (Ang) II alone, or with SIM for 28 d. Cardiac fibrosis, expressions of fibrosis-associated proteins and mRNAs, and collagen fiber arrangement and deposition were examined. Protein expressions in exosomes isolated from Ang II-treated cardiomyocytes (CMs) were evaluated using nano-ultra-performance liquid chromatographic system, combined with tandem mass spectrometry. Transformation of fibroblasts to myofibroblasts was evaluated using scanning electron and confocal microscopy, and migration assays. Our results showed that SIM attenuated in vivo expression of collagen and collagen-associated protein, as well as collagen deposition, and cardiac fibrosis. The statin also upregulated decorin and downregulated periostin in CM-derived exosomes. Furthermore, it suppressed Ang II-induced transformation of fibroblast to myofibroblast, as well as fibroblast migration. Exosome-mediated cell-cell communication within the cardiac tissue critically regulated cardiac fibrosis. Specifically, SIM regulated the release of CM exosomes, and attenuated Ang II-induced cardiac fibrosis, highlighting its potential as a novel therapy for cardiac fibrosis.
Keywords: cardiac fibrosis; decorin; exosomes; periostin; statins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes.J Mol Cell Cardiol. 2015 Dec;89(Pt B):268-79. doi: 10.1016/j.yjmcc.2015.10.022. Epub 2015 Oct 20. J Mol Cell Cardiol. 2015. PMID: 26497614 Free PMC article.
-
Epididymal white adipose tissue promotes angiotensin II-induced cardiac fibrosis in an exosome-dependent manner.Transl Res. 2022 Oct;248:51-67. doi: 10.1016/j.trsl.2022.05.004. Epub 2022 May 21. Transl Res. 2022. PMID: 35609783
-
Impaired cardiomyocytes accelerate cardiac hypertrophy and fibrosis by delivering exosomes containing Shh/N-Shh/Gli1 in angiotensin II infused mice.Heliyon. 2024 Oct 12;10(20):e39332. doi: 10.1016/j.heliyon.2024.e39332. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640644 Free PMC article.
-
Cardiac Fibroblasts and Cardiac Fibrosis: Precise Role of Exosomes.Front Cell Dev Biol. 2019 Dec 4;7:318. doi: 10.3389/fcell.2019.00318. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31867328 Free PMC article. Review.
-
Diagnostic and Therapeutic Properties of Exosomes in Cardiac Fibrosis.Front Cell Dev Biol. 2022 Jul 4;10:931082. doi: 10.3389/fcell.2022.931082. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35859903 Free PMC article. Review.
Cited by
-
Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer.Mol Cancer. 2022 Nov 1;21(1):207. doi: 10.1186/s12943-022-01671-0. Mol Cancer. 2022. PMID: 36320056 Free PMC article. Review.
-
Roles and Clinical Applications of Exosomes in Cardiovascular Disease.Biomed Res Int. 2020 Jun 10;2020:5424281. doi: 10.1155/2020/5424281. eCollection 2020. Biomed Res Int. 2020. PMID: 32596327 Free PMC article. Review.
-
Targeting the Tumor Microenvironment: A Literature Review of the Novel Anti-Tumor Mechanism of Statins.Front Oncol. 2021 Nov 11;11:761107. doi: 10.3389/fonc.2021.761107. eCollection 2021. Front Oncol. 2021. PMID: 34858839 Free PMC article. Review.
-
Extracellular Vesicles in Organ Fibrosis: Mechanisms, Therapies, and Diagnostics.Cells. 2021 Jun 25;10(7):1596. doi: 10.3390/cells10071596. Cells. 2021. PMID: 34202136 Free PMC article. Review.
-
The role of exosomes in immunopathology and potential therapeutic implications.Cell Mol Immunol. 2025 Sep;22(9):975-995. doi: 10.1038/s41423-025-01323-5. Epub 2025 Jul 14. Cell Mol Immunol. 2025. PMID: 40659888 Free PMC article. Review.
References
Grants and funding
- MOST104-2314-B-037-087/Ministry of Science and Technology, Taiwan
- MOST105-2314-B-037-046/Ministry of Science and Technology, Taiwan
- MOST104-2314-B-037-081-MY2/Ministry of Science and Technology, Taiwan
- KMUH102-2M18/Kaohsiung Medical University Chung-Ho Memorial Hospital
- KMTTH100-018/Kaohsiung Municipal Ta-Tung Hospital Research Foundation
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

