Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial
- PMID: 31167654
- PMCID: PMC6551895
- DOI: 10.1186/s12933-019-0871-8
Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial
Abstract
Background: The SUSTAIN 6 trial demonstrated that once-weekly semaglutide (0.5 and 1.0 mg) significantly reduced major adverse cardiovascular (CV) events (MACE) vs placebo in subjects with type 2 diabetes (T2D) and high CV risk. The effects of gender, age and baseline CV risk on outcomes are important considerations for further study.
Methods: Subjects were grouped according to gender, age (50-65 years and > 65 years), and CV risk profile at baseline (prior myocardial infarction [MI] or stroke vs no prior MI or stroke, and established CV disease [CVD] vs CV risk factors alone, including subjects with chronic kidney disease). Time to MACE and its individual components (CV death, nonfatal MI, nonfatal stroke), hospitalization for unstable angina or heart failure, and revascularization (coronary and peripheral) were analyzed for all subgroups. Additional analyses were performed for gender and age to investigate change from baseline in HbA1c and body weight, as well as tolerability.
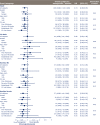
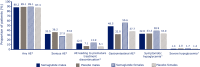
Results: A total of 3297 subjects were included. The majority of subjects (60.7%) were male; 43% were > 65 years of age; 41.5% had a history of MI or stroke; and 76.8% had established CVD. Compared with placebo, semaglutide reduced the risk of the first occurrence of MACE and each MACE component consistently across all subgroups (gender, age, and baseline CV risk profile). Revascularizations, HbA1c and body weight were also reduced consistently across all subgroups compared with placebo. Gastrointestinal adverse events in all treatment groups were more common among women than men, but rates of premature treatment discontinuation were similar for both genders.
Conclusions: In this post hoc analysis of SUSTAIN 6, once-weekly semaglutide vs placebo reduced the risk of MACE in all subjects included in the trial, regardless of gender, age, or baseline CV risk profile. Trial registry Clinicaltrials.gov, Identifying number: NCT01720446, Date of registration: October 29, 2012.
Keywords: Age; Baseline cardiovascular risk; Cardiovascular events; Cardiovascular outcome trial; Gender; SUSTAIN 6; Semaglutide; Type 2 diabetes.
Conflict of interest statement
During the conduct of the study, L.A.L. received research grants to his institution from Novo Nordisk. Outside of the submitted work, he has received grants and/or personal fees for advisory panels, speakers’ bureaus, and research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Merck, Novo Nordisk, and Sanofi. He also reports research grants from GlaxoSmithKline and personal fees for advisory panels from Servier.
Outside the submitted work, S.C.B. has received honoraria, teaching, and research sponsorship/grants from Abbott, AstraZeneca, Boehringer Ingelheim, Cellnovo, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi-aventis, Cardiff University, Doctors.net, Elsevier, Onmedica, Omnia-Med, Medscape, All-Wales Medicines Strategy Group, National Institute for Health and Care Excellence (NICE) UK, and Glycosmedia.
Outside the submitted work, I.H. reports research grants and/or personal fees from AstraZeneca/Bristol-Myers Squibb, Boehringer Ingelheim, Dexcom, Eli Lilly, GlaxoSmithKline, Insulet Corp, Janssen Pharmaceuticals, Lexicon Pharmaceuticals, Medtronic, Merck & Co Inc, Population Health Research Institute, Roche Pharma, Sanofi, and Takeda.
E.J. reports grants and personal fees from Novo Nordisk during the conduct of the study. He also reports grants and personal fees as a consultant and/or speaker from Amgen, AstraZeneca, Boehringer Ingelheim, Faes Farma, Fresenius, Janssen Pharmaceuticals, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi, Shire, and UCB.
Outside of the submitted work, S.M. has received honoraria and/or lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Meyers Squibb, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi Aventis. He has also received a research grant from Novo Nordisk and Boehringer Ingelheim.
T.G., T.H., and I.H. report that they are full-time employees of Novo Nordisk A/S.
I.L. received research grants to her institution and consulting fees from Novo Nordisk during the conduct of the study. Outside the submitted work, she has received grants and/or personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, GI Dynamics, Intarcia, Mannkind, Merck, Mylan, Novartis, NovoNordisk, Pfizer, Sanofi, TARGETPharma, and Valeritas.
Figures



References
-
- American Diabetes Association Standards of medical care in diabetes 2018. Diabetes Care. 2018;41(Suppl 1):S1–S135. - PubMed
-
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. - DOI - PMC - PubMed
-
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;2018(41):2669–2701. doi: 10.2337/dci18-0033. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

