The WT1-BASP1 complex is required to maintain the differentiated state of taste receptor cells
- PMID: 31167803
- PMCID: PMC6555901
- DOI: 10.26508/lsa.201800287
The WT1-BASP1 complex is required to maintain the differentiated state of taste receptor cells
Abstract
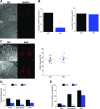
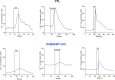
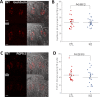
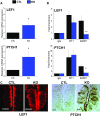
WT1 is a transcriptional activator that controls the boundary between multipotency and differentiation. The transcriptional cofactor BASP1 binds to WT1, forming a transcriptional repressor complex that drives differentiation in cultured cells; however, this proposed mechanism has not been demonstrated in vivo. We used the peripheral taste system as a model to determine how BASP1 regulates the function of WT1. During development, WT1 is highly expressed in the developing taste cells while BASP1 is absent. By the end of development, BASP1 and WT1 are co-expressed in taste cells, where they both occupy the promoter of WT1 target genes. Using a conditional BASP1 mouse, we demonstrate that BASP1 is critical to maintain the differentiated state of adult taste cells and that loss of BASP1 expression significantly alters the composition and function of these cells. This includes the de-repression of WT1-dependent target genes from the Wnt and Shh pathways that are normally only transcriptionally activated by WT1 in the undifferentiated taste cells. Our results uncover a central role for the WT1-BASP1 complex in maintaining cell differentiation in vivo.
© 2019 Gao et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
