Single-centre, triple-blinded, randomised, 1-year, parallel-group, superiority study to compare the effects of Roux-en-Y gastric bypass and sleeve gastrectomy on remission of type 2 diabetes and β-cell function in subjects with morbid obesity: a protocol for the Obesity s urg e ry in Tøns berg (O seberg) study
- PMID: 31167860
- PMCID: PMC6561424
- DOI: 10.1136/bmjopen-2018-024573
Single-centre, triple-blinded, randomised, 1-year, parallel-group, superiority study to compare the effects of Roux-en-Y gastric bypass and sleeve gastrectomy on remission of type 2 diabetes and β-cell function in subjects with morbid obesity: a protocol for the Obesity s urg e ry in Tøns berg (O seberg) study
Abstract
Introduction: Bariatric surgery is increasingly recognised as an effective treatment option for subjects with type 2 diabetes and obesity; however, there is no conclusive evidence on the superiority of Roux-en-Y gastric bypass or sleeve gastrectomy. The Oseberg study was designed to compare the effects of gastric bypass and sleeve gastrectomy on remission of type 2 diabetes and β-cell function.
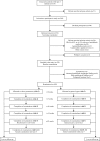
Methods and analysis: Single-centre, randomised, triple-blinded, two-armed superiority trial carried out at the Morbid Obesity Centre at Vestfold Hospital Trust in Norway. Eligible patients with type 2 diabetes and obesity were randomly allocated in a 1:1 ratio to either gastric bypass or sleeve gastrectomy. The primary outcome measures are (1) the proportion of participants with complete remission of type 2 diabetes (HbA1c≤6.0% in the absence of blood glucose-lowering pharmacologic therapy) and (2) β-cell function expressed by the disposition index (calculated using the frequently sampled intravenous glucose tolerance test with minimal model analysis) 1 year after surgery.
Ethics and dissemination: The protocol of the current study was reviewed and approved by the regional ethics committee on 12 September 2012 (ref: 2012/1427/REK sør-øst B). The results will be disseminated to academic and health professional audiences and the public via publications in international peer-reviewed journals and conferences. Participants will receive a summary of the main findings.
Trial registration number: NCT01778738;Pre-results.
Keywords: gastric bypass; morbid obesity; randomised controlled trial; sleeve gastrectomy; type 2 diabetes; β-cell function.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
References
-
- American Society for MaBS. Estimate of Bariatric Surgery Numbers, 2011-2016. 2016. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials