Autoantibodies Directed Toward a Novel IA-2 Variant Protein Enhance Prediction of Type 1 Diabetes
- PMID: 31167877
- PMCID: PMC6702638
- DOI: 10.2337/db18-1351
Autoantibodies Directed Toward a Novel IA-2 Variant Protein Enhance Prediction of Type 1 Diabetes
Abstract
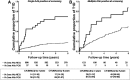
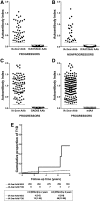
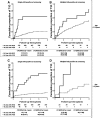
We identified autoantibodies (AAb) reacting with a variant IA-2 molecule (IA-2var) that has three amino acid substitutions (Cys27, Gly608, and Pro671) within the full-length molecule. We examined IA-2var AAb in first-degree relatives of type 1 diabetes (T1D) probands from the TrialNet Pathway to Prevention Study. The presence of IA-2var-specific AAb in relatives was associated with accelerated progression to T1D in those positive for AAb to GAD65 and/or insulin but negative in the standard test for IA-2 AAb. Furthermore, relatives with single islet AAb (by traditional assays) and carrying both IA-2var AAb and the high-risk HLA-DRB1*04-DQB1*03:02 haplotype progress rapidly to onset of T1D. Molecular modeling of IA-2var predicts that the genomic variation that alters the three amino acids induces changes in the three-dimensional structure of the molecule, which may lead to epitope unmasking in the IA-2 extracellular domain. Our observations suggest that the presence of AAb to IA-2var would identify high-risk subjects who would benefit from participation in prevention trials who have one islet antibody by traditional testing and otherwise would be misclassified as "low risk" relatives.
© 2019 by the American Diabetes Association.
Figures



References
-
- Roep BO, Peakman M. Diabetogenic T lymphocytes in human type 1 diabetes. Curr Opin Immunol 2011;23:746–753 - PubMed
-
- Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol 1994;13:505–514 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK085476/DK/NIDDK NIH HHS/United States
- R01 DK053456/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK103282/DK/NIDDK NIH HHS/United States
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- R01 DK032083/DK/NIDDK NIH HHS/United States
- R01 DK108868/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK106984/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK107013/DK/NIDDK NIH HHS/United States
- U01 DK103266/DK/NIDDK NIH HHS/United States
- U01 DK107014/DK/NIDDK NIH HHS/United States
- U01 DK106994/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- UC4 DK097835/DK/NIDDK NIH HHS/United States
- U01 DK103180/DK/NIDDK NIH HHS/United States
- U01 DK085465/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

