Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance
- PMID: 31167878
- PMCID: PMC6702639
- DOI: 10.2337/db18-0927
Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance
Abstract
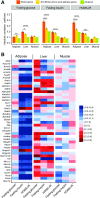
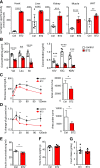
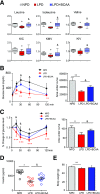
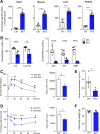
Recent studies implicate a strong association between elevated plasma branched-chain amino acids (BCAAs) and insulin resistance (IR). However, a causal relationship and whether interrupted BCAA homeostasis can serve as a therapeutic target for diabetes remain to be established experimentally. In this study, unbiased integrative pathway analyses identified a unique genetic link between obesity-associated IR and BCAA catabolic gene expression at the pathway level in human and mouse populations. In genetically obese (ob/ob) mice, rate-limiting branched-chain α-keto acid (BCKA) dehydrogenase deficiency (i.e., BCAA and BCKA accumulation), a metabolic feature, accompanied the systemic suppression of BCAA catabolic genes. Restoring BCAA catabolic flux with a pharmacological inhibitor of BCKA dehydrogenase kinase (BCKDK) ( a suppressor of BCKA dehydrogenase) reduced the abundance of BCAA and BCKA and markedly attenuated IR in ob/ob mice. Similar outcomes were achieved by reducing protein (and thus BCAA) intake, whereas increasing BCAA intake did the opposite; this corroborates the pathogenic roles of BCAAs and BCKAs in IR in ob/ob mice. Like BCAAs, BCKAs also suppressed insulin signaling via activation of mammalian target of rapamycin complex 1. Finally, the small-molecule BCKDK inhibitor significantly attenuated IR in high-fat diet-induced obese mice. Collectively, these data demonstrate a pivotal causal role of a BCAA catabolic defect and elevated abundance of BCAAs and BCKAs in obesity-associated IR and provide proof-of-concept evidence for the therapeutic validity of manipulating BCAA metabolism for treating diabetes.
© 2019 by the American Diabetes Association.
Figures



References
-
- Felig P, Marliss E, Cahill GFJ Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811–816 - PubMed
-
- Lu J, Xie G, Jia W, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med 2013;7:53–59 - PubMed
-
- Lu Y, Wang Y, Ong C-N, et al. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016;59:2349–2359 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

