Ambient air pollution and pulmonary vascular volume on computed tomography: the MESA Air Pollution and Lung cohort studies
- PMID: 31167881
- PMCID: PMC6910868
- DOI: 10.1183/13993003.02116-2018
Ambient air pollution and pulmonary vascular volume on computed tomography: the MESA Air Pollution and Lung cohort studies
Abstract
Background: Air pollution alters small pulmonary vessels in animal models. We hypothesised that long-term ambient air pollution exposure would be associated with differences in pulmonary vascular volumes in a population-based study.
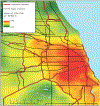
Methods: The Multi-Ethnic Study of Atherosclerosis recruited adults in six US cities. Personalised long-term exposures to ambient black carbon, nitrogen dioxide (NO2), oxides of nitrogen (NO x ), particulate matter with a 50% cut-off aerodynamic diameter of <2.5 μm (PM2.5) and ozone were estimated using spatiotemporal models. In 2010-2012, total pulmonary vascular volume was measured as the volume of detectable pulmonary arteries and veins, including vessel walls and luminal blood volume, on noncontrast chest computed tomography (TPVVCT). Peripheral TPVVCT was limited to the peripheral 2 cm to isolate smaller vessels. Linear regression adjusted for demographics, anthropometrics, smoking, second-hand smoke, renal function and scanner manufacturer.
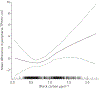
Results: The mean±sd age of the 3023 participants was 69.3±9.3 years; 46% were never-smokers. Mean exposures were 0.80 μg·m-3 black carbon, 14.6 ppb NO2 and 11.0 μg·m-3 ambient PM2.5. Mean±sd peripheral TPVVCT was 79.2±18.2 cm3 and TPVVCT was 129.3±35.1 cm3. Greater black carbon exposure was associated with a larger peripheral TPVVCT, including after adjustment for city (mean difference 0.41 (95% CI 0.03-0.79) cm3 per interquartile range; p=0.036). Associations for peripheral TPVVCT with NO2 were similar but nonsignificant after city adjustment, while those for PM2.5 were of similar magnitude but nonsignificant after full adjustment. There were no associations for NO x or ozone, or between any pollutant and TPVVCT.
Conclusions: Long-term black carbon exposure was associated with a larger peripheral TPVVCT, suggesting diesel exhaust may contribute to remodelling of small pulmonary vessels in the general population.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: C.P. Aaron reports grants from NIH, during the conduct of the study; grants from Alpha1 Foundation and Stony Wold-Herbert Fund, outside the submitted work. Conflict of interest: E.A. Hoffman reports grants from NIH, during the conduct of the study; grants from NIH, outside the submitted work; and is a founder and shareholder of VIDA Diagnostics, from whom software was utilised for the data analysis. Conflict of interest: S.M. Kawut reports grants from NIH, during the conduct of the study; grants from NIH, Actelion, United Therapeutic, Gilead, Lung Biotech, Pfizer, Ikaria, Merck, Bayer, Pulmonary Hypertension Association and GeNO, travel reimbursement from American College of Chest Physicians and American Thoracic Society, and personal fees from European Respiratory Journal, outside the submitted work. Conflict of interest: J.H.M. Austin reports grants from NIH, during the conduct of the study; personal fees from PulmonX, outside the submitted work. Conflict of interest: M. Budoff reports grants from NIH, during the conduct of the study; grants from GE, outside the submitted work. Conflict of interest: E.D. Michos reports personal fees from Siemens Healthcare Diagnostics, outside the submitted work. Conflict of interest: K. Hinckley Stukovsky reports salary support from Cystic Fibrosis Foundation, outside the submitted work. Conflict of interest: C. Sack has nothing to disclose. Conflict of interest: A.A. Szpiro has nothing to disclose. Conflict of interest: K.D. Watson reports grants from NIH, during the conduct of the study. Conflict of interest: J.D. Kaufman reports grants from US EPA, during the conduct of the study. Conflict of interest: R.G. Barr reports grants from NIH, during the conduct of the study; grants from NIH, Alpha1 Foundation and COPD Foundation, outside the submitted work.
Figures

References
-
- Lippman M, Leikauf GD. Introduction and background In: Lippman M, ed. Environmental Toxicants. 3rd Edn. New York, Wiley, 2009; pp. 1–38.
-
- Weibel ER. Morphometry of the Human Lung. Berlin, Springer, 1963.
-
- Liu J, Ye X, Ji D, et al. Diesel exhaust inhalation exposure induces pulmonary arterial hypertension in mice. Environ Pollut 2018; 237: 747–755. - PubMed
-
- Lemos M, Mohallen SV, Macchione M, et al. Chronic exposure to urban air pollution induces structural alterations in murine pulmonary and coronary arteries. Inhal Toxicol 2006; 18: 247–253. - PubMed
-
- Rivero DH, Soares SR, Lorenzi-Filho G, et al. Acute cardiopulmonary alterations induced by fine particulate matter of Sao Paulo, Brazil. Toxicol Sci 2005; 85: 898–905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 HC095161/HL/NHLBI NIH HHS/United States
- S10 OD018526/OD/NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- K23 HL141651/HL/NHLBI NIH HHS/United States
- R01 HL077612/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- R01 HL093081/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- K24 HL103844/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
