On the occurrence of cytochrome P450 in viruses
- PMID: 31167942
- PMCID: PMC6589655
- DOI: 10.1073/pnas.1901080116
On the occurrence of cytochrome P450 in viruses
Abstract
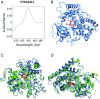
Genes encoding cytochrome P450 (CYP; P450) enzymes occur widely in the Archaea, Bacteria, and Eukarya, where they play important roles in metabolism of endogenous regulatory molecules and exogenous chemicals. We now report that genes for multiple and unique P450s occur commonly in giant viruses in the Mimiviridae, Pandoraviridae, and other families in the proposed order Megavirales. P450 genes were also identified in a herpesvirus (Ranid herpesvirus 3) and a phage (Mycobacterium phage Adler). The Adler phage P450 was classified as CYP102L1, and the crystal structure of the open form was solved at 2.5 Å. Genes encoding known redox partners for P450s (cytochrome P450 reductase, ferredoxin and ferredoxin reductase, and flavodoxin and flavodoxin reductase) were not found in any viral genome so far described, implying that host redox partners may drive viral P450 activities. Giant virus P450 proteins share no more than 25% identity with the P450 gene products we identified in Acanthamoeba castellanii, an amoeba host for many giant viruses. Thus, the origin of the unique P450 genes in giant viruses remains unknown. If giant virus P450 genes were acquired from a host, we suggest it could have been from an as yet unknown and possibly ancient host. These studies expand the horizon in the evolution and diversity of the enormously important P450 superfamily. Determining the origin and function of P450s in giant viruses may help to discern the origin of the giant viruses themselves.
Keywords: cytochrome P450; domains of life; evolution; redox partner; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Klingenberg M., Pigments of rat liver microsomes. Arch. Biochem. Biophys. 75, 376–386 (1958). - PubMed
-
- Omura T., Sato R., A new cytochrome in liver microsomes. J. Biol. Chem. 237, 1375–1376 (1962). - PubMed
-
- Estabrook R. W., Cooper D. Y., Rosenthal O., The light reversible carbon monoxide inhibition of the steroid C21-hydroxylase system of the adrenal cortex. Biochem. Z. 338, 741–755 (1963). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions

