Individual brain organoids reproducibly form cell diversity of the human cerebral cortex
- PMID: 31168097
- PMCID: PMC6906116
- DOI: 10.1038/s41586-019-1289-x
Individual brain organoids reproducibly form cell diversity of the human cerebral cortex
Abstract
Experimental models of the human brain are needed for basic understanding of its development and disease1. Human brain organoids hold unprecedented promise for this purpose; however, they are plagued by high organoid-to-organoid variability2,3. This has raised doubts as to whether developmental processes of the human brain can occur outside the context of embryogenesis with a degree of reproducibility that is comparable to the endogenous tissue. Here we show that an organoid model of the dorsal forebrain can reliably generate a rich diversity of cell types appropriate for the human cerebral cortex. We performed single-cell RNA-sequencing analysis of 166,242 cells isolated from 21 individual organoids, finding that 95% of the organoids generate a virtually indistinguishable compendium of cell types, following similar developmental trajectories and with a degree of organoid-to-organoid variability comparable to that of individual endogenous brains. Furthermore, organoids derived from different stem cell lines show consistent reproducibility in the cell types produced. The data demonstrate that reproducible development of the complex cellular diversity of the central nervous system does not require the context of the embryo, and that establishment of terminal cell identity is a highly constrained process that can emerge from diverse stem cell origins and growth environments.
Conflict of interest statement
The authors declare no competing interests.
Figures
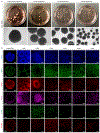







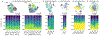
References
-
- Quadrato G, Brown J & Arlotta P The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med 22, 1220–1228 (2016). - PubMed
Supplementary References
-
- Velasco S, Paulsen B & Arlotta P Highly reproducible human brain organoids recapitulate cerebral cortex cellular diversity. Nat. Protoc. Exch (2019).
-
- Quadrato G, Sherwood JL & Arlotta P Long term culture and electrophysiological characterization of human brain organoids. Protoc. Exch (2017). doi: 10.1038/protex.2017.049 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

