High incidence of atrial fibrillation in patients treated with ibrutinib
- PMID: 31168393
- PMCID: PMC6519413
- DOI: 10.1136/openhrt-2019-001049
High incidence of atrial fibrillation in patients treated with ibrutinib
Abstract
Objective: Atrial fibrillation (AF) is one of the most common side effects of ibrutinib, a drug that has dramatically improved the prognosis of chronic B-cell malignancies such as chronic lymphocytic leukaemia (CLL). The true incidence of ibrutinib-related AF (IRAF) is not well known and its therapeutic management poses unique challenges especially due to the inherent risk of bleeding. We aimed to determine the incidence and predictors of IRAF, and to analyse its management and outcome.
Methods: A standardised monitoring was applied at two cardio-oncology clinics in consecutive patients referred before and during ibrutinib therapy. The primary endpoint was the incidence of IRAF. The excess of AF incidence with ibrutinib was studied by comparing the incidence of IRAF with the expected incidence of AF in general population and in patients with CLL not exposed to ibrutinib.
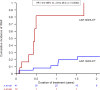
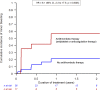
Results: 53 patients were included. The incidence of IRAF was 38% at 2 years and the risk was 15-fold higher than the AF risk in both the general population and patients with CLL not exposed to ibrutinib (p<0.0001). The majority of cases occurred in asymptomatic patients within the first 6 months. Left atrial volume index ≥40 mL/m2 at treatment initiation identified patients at high risk of developing IRAF. No major bleeding events occurred in patients on ibrutinib, although the majority of patients with IRAF were treated with anticoagulants.
Conclusions: This cardio-oncology study showed that the risk of IRAF was much higher than previously reported. The majority of cases occurred in asymptomatic patients justifying close monitoring.
Keywords: atrial fibrillation; cardio-oncology; cardiotoxicity; ibrutinib.
Conflict of interest statement
Competing interests: JC received received modest consultant and lecture fees from MSD, Janssen, Merck, Novartis, Astra-Zeneca. FT received grants from Fédération Francaise de Cardiologie, Ministère français de la Santé, Fondation Coeur et Recherche, Ligue contre le Cancer, Assistance Publique – Hôpitaux de Marseille, Vifor Pharma. FT exercised an expert activity with Institut Nationale du Cancer. FT received personal fees for lectures and speakers bureaus from Abbott, Novartis, Amgen, Janssen-Cilag, Merck Sharp and Dohme, Bristol-Myers Squibb, Pfizer, Roche, Vifor Pharma, Sanofi, Astra-Zeneca. FT received non-financial support for travel and lunch paid from Abbott, Novartis, Amgen, Janssen-Cilag, Merck Sharp and Dohme, Bristol-Myers Squibb, Pfizer, Roche, Vifor Pharma, Sanofi, Astra-Zeneca, Servier, Sorin, Boston Scientific, Actelion, Bayer, Biotronik, Boehinger Ingelheim, The Medecines Compagny, Orion Pharma, Correvio, Daiichi Sankyo, St Jude Medical, Lilly, Zoll Medical, LivaNova, Medtronic, Sorin, Philips, Genzyme, Icomed, Leo Pharma, Mylan Medical, Preciphar, Resmed. AC received modest consultant and lecture fees from Astra-Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, and Sanofi-Aventis.Stephane Ederhy received modest consultant and lecture fees from Lilly, Daiichy-Sankyo, Celgene, Pfizer, Esperare, Bristol-Myers Squibb, Janssen, Philips Healthcare, Bayer, Novartis, Amgen, Ipsen.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Medical
