Cell-Penetrating Dynamic-Covalent Benzopolysulfane Networks
- PMID: 31168906
- PMCID: PMC6618005
- DOI: 10.1002/anie.201905003
Cell-Penetrating Dynamic-Covalent Benzopolysulfane Networks
Abstract
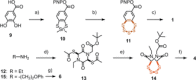
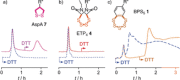
Cyclic oligochalcogenides (COCs) are emerging as promising systems to penetrate cells. Clearly better than and different to the reported diselenolanes and epidithiodiketopiperazines, we introduce the benzopolysulfanes (BPS), which show efficient delivery, insensitivity to inhibitors of endocytosis, and compatibility with substrates as large as proteins. This high activity coincides with high reactivity, selectively toward thiols, exceeding exchange rates of disulfides under tension. The result is a dynamic-covalent network of extreme sulfur species, including cyclic oligomers, from dimers to heptamers, with up to nineteen sulfurs in the ring. Selection from this unfolding adaptive network then yields the reactivities and selectivities needed to access new uptake pathways. Contrary to other COCs, BPS show high retention on thiol affinity columns. The identification of new modes of cell penetration is important because they promise new solutions to challenges in delivery and beyond.
Keywords: adaptive networks; cellular uptake; cyclic oligochalcogenides; dynamic-covalent chemistry; polysulfanes.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Steudel R., Chem. Rev. 2002, 102, 3905–3946. - PubMed
-
- None
-
- Konstantinova L. S., Rakitin O. A., Rees C. W., Chem. Rev. 2004, 104, 2617–2630; - PubMed
-
- Münchberg U., Anwar A., Mecklenburg S., Jacob C., Org. Biomol. Chem. 2007, 5, 1505–1518; - PubMed
-
- Jiang C.-S., Müller W. E. G., Schröder H. C., Guo Y.-W., Chem. Rev. 2012, 112, 2179–2207; - PubMed
Publication types
Grants and funding
- 200020 175486/Swiss NSF/International
- Molecular Systems Engineering/National Centre of Competence in Research (NCCR)/International
- Chemical Biology/National Centre of Competence in Research (NCCR)/International
- University of Geneva/International
- 740288/Marie Sklodowska-Curie Actions Individual Fellowship/International
LinkOut - more resources
Full Text Sources

