Advances in Biomaterials and Technologies for Vascular Embolization
- PMID: 31168915
- PMCID: PMC7014563
- DOI: 10.1002/adma.201901071
Advances in Biomaterials and Technologies for Vascular Embolization
Abstract
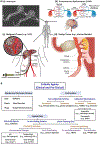
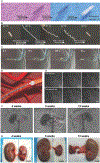
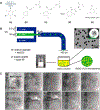
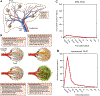
Minimally invasive transcatheter embolization is a common nonsurgical procedure in interventional radiology used for the deliberate occlusion of blood vessels for the treatment of diseased or injured vasculature. A wide variety of embolic agents including metallic coils, calibrated microspheres, and liquids are available for clinical practice. Additionally, advances in biomaterials, such as shape-memory foams, biodegradable polymers, and in situ gelling solutions have led to the development of novel preclinical embolic agents. The aim here is to provide a comprehensive overview of current and emerging technologies in endovascular embolization with respect to devices, materials, mechanisms, and design guidelines. Limitations and challenges in embolic materials are also discussed to promote advancement in the field.
Keywords: catheter-based delivery; embolic agents; interventional radiology; minimally invasive surgery; vascular embolization.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest
RO and AK have recently launched a joint start-up Obsidio, Inc. in the field of vascular embolization.
Figures












References
-
- Leyon JJ, Littlehales T, Rangarajan B, Hoey ET, Ganeshan A, Curr. Probl. Diagn. Radiol 2014, 43, 35. - PubMed
-
- Interventional Radiology, Williams and Wilkins, Baltimore: 1997;
- Luessenhop AJ, Spence WT, JAMA, J. Am. Med. Assoc 1960, 172, 1153; - PubMed
- Dawbarn RHM, J. Am. Med. Assoc 1904, 43, 0792.
-
- Brooks B, South. Med. J 1930, 23, 100.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

