Antibodies and vaccines against Middle East respiratory syndrome coronavirus
- PMID: 31169078
- PMCID: PMC6567157
- DOI: 10.1080/22221751.2019.1624482
Antibodies and vaccines against Middle East respiratory syndrome coronavirus
Abstract
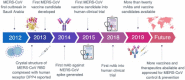
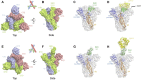
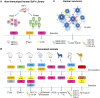
The Middle East respiratory syndrome coronavirus (MERS-CoV) has spread through 27 countries and infected more than 2,200 people since its first outbreak in Saudi Arabia in 2012. The high fatality rate (35.4%) of this novel coronavirus and its persistent wide spread infectiousness in animal reservoirs have generated tremendous global public health concern. However, no licensed therapeutic agents or vaccines against MERS-CoV are currently available and only a limited few have entered clinical trials. Among all the potential targets of MERS-CoV, the spike glycoprotein (S) has been the most well-studied due to its critical role in mediating viral entry and in inducing a protective antibody response in infected individuals. The most notable studies include the recent discoveries of monoclonal antibodies and development of candidate vaccines against the S glycoprotein. Structural characterization of MERS-CoV S protein bound with these monoclonal antibodies has provided insights into the mechanisms of humoral immune responses against MERS-CoV infection. The current review aims to highlight these developments and discuss possible hurdles and strategies to translate these discoveries into ultimate medical interventions against MERS-CoV infection.
Keywords: Coronavirus; MERS-CoV; monoclonal antibody; spike glycoprotein; vaccine.
Figures


References
-
- Hamre D, Procknow JJ.. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966 Jan;121(1):190–193. PubMed PMID: 4285768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
