Susceptibility to adverse drug reactions
- PMID: 31169324
- PMCID: PMC6783620
- DOI: 10.1111/bcp.14015
Susceptibility to adverse drug reactions
Abstract
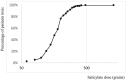
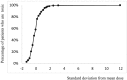
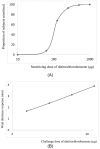
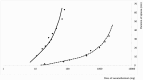
The pharmacological effects of a drug depend on its concentration at the site of action, and therefore on the concentration in blood and on the dose. The relationship between the concentration or dose and the corresponding effect can usually be represented mathematically as a rectangular hyperbola; when effect is plotted against log concentration or log dose, the curve is sigmoidal. Inevitably, the effect size and the doses causing benefit and harm will differ among individuals, since they are biological phenomena: some individuals are more likely than others to suffer harm at any given dose. Some harmful effects can occur at much lower doses than those used in therapeutics; that is, the log dose-response curve for harm lies far to the left of the log dose-response curve for benefit. Those who suffer such reactions are hypersusceptible. When the dose-response curves for harm and therapeutic effect are in the same range, dose cannot separate the harmful effects from the therapeutic effects, and adverse reactions are collateral. Toxic effects occur when harmful doses are above the doses needed for benefit. In this review we consider factors that influence a subject's susceptibility to adverse drug reactions. Determinants of susceptibility include Immunological, Genetic, demographic (Age and Sex), Physiological and Exogenous factors (drug-drug interactions, for example), and Diseases and disorders such as renal failure, giving the mnemonic I GASPED. Some susceptibility factors are discrete (for example, all-or-none) and some are continuous; susceptibility can therefore be discrete or continuous; and the factors can interact to determine a person's overall susceptibility to harm.
Keywords: adverse drug reactions; genetic polymorphisms; pharmacodynamics; pharmacokinetics; prescribing.
© 2019 The British Pharmacological Society.
Conflict of interest statement
This work was unfunded. Both R.E.F. and J.K.A. have provided expert advice on ADRs and have sometimes received payment for this advice.
Figures
Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Harms from medicines: inevitable, in error or intentional.Br J Clin Pharmacol. 2014 Mar;77(3):403-9. doi: 10.1111/bcp.12156. Br J Clin Pharmacol. 2014. PMID: 23683079 Free PMC article. Review.
-
The law of mass action and the pharmacological concentration-effect curve: resolving the paradox of apparently non-dose-related adverse drug reactions.Br J Clin Pharmacol. 2016 Jan;81(1):56-61. doi: 10.1111/bcp.12706. Epub 2015 Oct 26. Br J Clin Pharmacol. 2016. PMID: 26119837 Free PMC article. Review.
-
Mechanistic basis of adverse drug reactions: the perils of inappropriate dose schedules.Expert Opin Drug Saf. 2005 Jan;4(1):103-28. doi: 10.1517/14740338.4.1.103. Expert Opin Drug Saf. 2005. PMID: 15709902 Review.
-
Clinical pharmacokinetics: current requirements and future perspectives from a regulatory point of view.Xenobiotica. 1993 Nov;23(11):1159-93. doi: 10.3109/00498259309059432. Xenobiotica. 1993. PMID: 8310705 Review.
Cited by
-
On precision dosing of oral small molecule drugs in oncology.Br J Clin Pharmacol. 2021 Feb;87(2):263-270. doi: 10.1111/bcp.14454. Epub 2020 Jul 17. Br J Clin Pharmacol. 2021. PMID: 32621551 Free PMC article. Review.
-
Statin doses after acute coronary syndrome.Aust Prescr. 2022 Apr;45(2):42. doi: 10.18773/austprescr.2022.020. Epub 2022 Apr 1. Aust Prescr. 2022. PMID: 35592374 Free PMC article. No abstract available.
-
Risk factors for adverse drug reactions associated with clopidogrel therapy.Open Med (Wars). 2022 Apr 7;17(1):694-701. doi: 10.1515/med-2021-0371. eCollection 2022. Open Med (Wars). 2022. PMID: 35480401 Free PMC article.
-
Clinical trial diversity: An opportunity for improved insight into the determinants of variability in drug response.Br J Clin Pharmacol. 2022 Jun;88(6):2700-2717. doi: 10.1111/bcp.15242. Epub 2022 Feb 17. Br J Clin Pharmacol. 2022. PMID: 35088432 Free PMC article. Review.
-
Precision medicine technology hype or reality? The example of computer-guided dosing.F1000Res. 2019 Oct 1;8:1709. doi: 10.12688/f1000research.20489.1. eCollection 2019. F1000Res. 2019. PMID: 31754426 Free PMC article.
References
-
- Aronson JK, Ferner RE. It does all depend on the dose. Understanding beneficial and adverse drug effects since 1864: clinical and experimental attitudes to the law of mass action and concentration–effect curves In: Grell O, Cunningham A, Arrizabalaga J, eds. It All Depends on the Dose': Poisons and Medicines in European History. Taylor & Francis Ltd. London: Imprint Routledge; 2018:210‐239.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

