Efficacy and safety assessment of S-1-based regimens comparing to intravenous fluorouracil-based ones in Asian patients with metastatic colorectal carcinoma: A system review and meta-analysis
- PMID: 31169742
- PMCID: PMC6571363
- DOI: 10.1097/MD.0000000000015999
Efficacy and safety assessment of S-1-based regimens comparing to intravenous fluorouracil-based ones in Asian patients with metastatic colorectal carcinoma: A system review and meta-analysis
Abstract
Background: We performed the present systematic review and meta-analysis to evaluate the efficacy and safety for S-1-based regimens comparing to intravenous fluorouracil-based ones in Asian patients with metastatic colorectal carcinoma (mCRC).
Methods: Eligible prospective and controlled randomized clinical trials (RCT) were included, of which data were extracted by inclusion criteria and exclusion ones. Odds ratio (OR) and Hazard ratio (HR) of outcomes including objective response rate (ORR), disease control rate (DCR), progressive-free survival (PFS), overall survival (OS), and adverse events (AEs) were explored for the final analysis between the 2 groups.
Results: A total of 23 eligible prospective, controlled RCTs including 2269 patients were enrolled for the pooled analysis. With the meta-analysis of available data, the results of the present research showed that there was no statistical difference on short-term efficacy including ORR (HR = 0.85, 95% CI: 0.71-1.01; P = .07) or DCR (HR = 0.88, 95% CI: 0.69-1.11; P = .27), as well as long-term efficacy including PFS (HR = 1.00, 95% CI: 0.90-1.11; P = .98) or OS (HR = 0.95, 95% CI: 0.82-1.10; P = .50). In addition, the incidences of AEs including leucopenia, neutropenia, and vomiting were statistically lower in S-1-based regimens comparing to intravenous fluorouracil-based ones, regardless of all grade or high grade (all P <.05). However, there were no significant differences detected among other AEs including anemia, thrombocytopenia, increased alanine aminotransferase concentration, stomatitis, anorexia, diarrhea, hand-foot syndrome (HFS), or sensory neuropathy among the 2 groups (all P >.05).
Conclusions: The present meta-analysis revealed that S-1-based regimens might be associated with comparable efficacy, as well as lower risk of leucopenia, neutropenia, and vomiting at all/high grade comparing to intravenous fluorouracil-based ones in Asian patients with mCRC.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


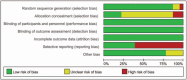




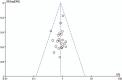
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer 2015;136:E359–386. - PubMed
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in china, 2015. CA Cancer J Clin 2016;66:115–32. - PubMed
-
- Yoshino T, Arnold D, Taniguchi H, et al. Pan-asian adapted esmo consensus guidelines for the management of patients with metastatic colorectal cancer: a jsmo-esmo initiative endorsed by csco, kaco, mos, sso and tos. Ann Oncol Off J Eur Soc Med Oncol 2018;29:44–70. - PubMed
-
- Nishina T, Azuma M, Nishikawa K, et al. Early tumor shrinkage and depth of response in patients with advanced gastric cancer: a retrospective analysis of a randomized phase iii study of first-line s-1 plus oxaliplatin vs. S-1 plus cisplatin. Gastric Cancer 2019;22:138–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

