International longitudinal registry of patients with atrial fibrillation and treated with rivaroxaban: RIVaroxaban Evaluation in Real life setting (RIVER)
- PMID: 31169831
- PMCID: PMC6482585
- DOI: 10.1186/s12959-019-0195-7
International longitudinal registry of patients with atrial fibrillation and treated with rivaroxaban: RIVaroxaban Evaluation in Real life setting (RIVER)
Abstract
Background: Real-world data on non-vitamin K oral anticoagulants (NOACs) are essential in determining whether evidence from randomised controlled clinical trials translate into meaningful clinical benefits for patients in everyday practice. RIVER (RIVaroxaban Evaluation in Real life setting) is an ongoing international, prospective registry of patients with newly diagnosed non-valvular atrial fibrillation (NVAF) and at least one investigator-determined risk factor for stroke who received rivaroxaban as an initial treatment for the prevention of thromboembolic stroke. The aim of this paper is to describe the design of the RIVER registry and baseline characteristics of patients with newly diagnosed NVAF who received rivaroxaban as an initial treatment.
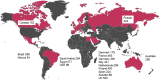
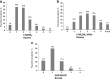
Methods and results: Between January 2014 and June 2017, RIVER investigators recruited 5072 patients at 309 centres in 17 countries. The aim was to enroll consecutive patients at sites where rivaroxaban was already routinely prescribed for stroke prevention. Each patient is being followed up prospectively for a minimum of 2-years. The registry will capture data on the rate and nature of all thromboembolic events (stroke / systemic embolism), bleeding complications, all-cause mortality and other major cardiovascular events as they occur. Data quality is assured through a combination of remote electronic monitoring and onsite monitoring (including source data verification in 10% of cases). Patients were mostly enrolled by cardiologists (n = 3776, 74.6%), by internal medicine specialists 14.2% (n = 718) and by primary care/general practice physicians 8.2% (n = 417). The mean (SD) age of the population was 69.5 (11.0) years, 44.3% were women. Mean (SD) CHADS2 score was 1.9 (1.2) and CHA2DS2-VASc scores was 3.2 (1.6). Almost all patients (98.5%) were prescribed with once daily dose of rivaroxaban, most commonly 20 mg (76.5%) and 15 mg (20.0%) as their initial treatment; 17.9% of patients received concomitant antiplatelet therapy. Most patients enrolled in RIVER met the recommended threshold for AC therapy (86.6% for 2012 ESC Guidelines, and 79.8% of patients according to 2016 ESC Guidelines).
Conclusions: The RIVER prospective registry will expand our knowledge of how rivaroxaban is prescribed in everyday practice and whether evidence from clinical trials can be translated to the broader cross-section of patients in the real world.
Trial registration: Unique identifier: NCT02444221. Registerd 14 May 2015; Retrospectively Registered.
Keywords: Anticoagulant; Antithrombotic; Atrial fibrillation; Outcomes; Registry; Rivaroxaban.
Conflict of interest statement
Independent ethics committee and hospital-based institutional review board approvals were obtained, as necessary, for the registry protocol.Not applicable.JB-W has received grants and personal fees from Bayer, Daiichi, Janssen, Pfizer and Portola and has received grants from Boehringer. AJC has received personal fees from Bayer, Boehringer Ingelheim, Pfizer/BMS, Daiichi Sankyo, Portola and Verseon and has received grants from Bayer, Boehringer Ingelheim, Pfizer/BMS and Daiichi Sankyo. KAAF has received grants and personal fees from Bayer/Janssen and AstraZeneca and personal fees from Sanofi/Regeneron and Verseon outside the submitted work. JYLH has received personal fee from AstraZeneca, Bayer, Boehringer Ingelheim, BMS/Pfizer, Daiichi Sankyo, Meda, Sanofi, and Servier. SH has received personal fees from Aspen, Bayer Healthcare, BMS/Pfizer, Daiichi-Sankyo, Portola and Sanofi. AGGT has received personal fees from Bayer Healthcare, Janssen Pharmaceutical Research & Development LLC, Astellas, Portola, and Takeda.SV has no disclosures. AKK has received research support from Bayer AG; personal fees from Bayer AG, Boehringer-Ingelheim Pharma, Daiichi Sankyo Europe, Janssen Pharma, Sanofi SA and Verseon.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0. - DOI - PubMed
-
- Beyer-Westendorf J, Ebertz F, Forster K, Gelbricht V, Michalski F, Kohler C, et al. Effectiveness and safety of dabigatran therapy in daily-care patients with atrial fibrillation. Results from the Dresden NOAC registry. Thromb Haemost. 2015;113(6):1247–1257. doi: 10.1160/TH14-11-0954. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

