Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list
- PMID: 31169941
- PMCID: PMC6816014
- DOI: 10.1111/apt.15326
Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list
Abstract
Background: Rifaximin reduces the risk of overt hepatic encephalopathy (HE) and is associated with significant reductions in hospitalisations and 30-day readmissions.
Aim: To examine the outcomes of patients listed for liver transplantation with a diagnosis of HE on rifaximin compared to those naïve to the drug.
Methods: Patient records of those listed for liver transplantation over a 2-year period were retrospectively reviewed. Patients were included if they had at least two episodes of overt HE resulting in hospitalisation or were encephalopathic at the time of assessment.
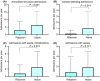
Results: Of the 622 patients listed for transplantation, 101 had HE. Sixty-six patients were treated with rifaximin and 35 were naïve at listing. The use of concurrent lactulose was not significantly different between groups. Median MELD score was similar (15 [14-16)] rifaximin-treated and 16 [14-18] rifaximin-naïve). Patients on the waiting list treated with rifaximin had reduced all-cause admissions, episodes of spontaneous bacterial peritonitis and variceal bleeding. Mean length of stay was 9 days (95% CI 6-12) in the rifaximin-treated group vs 14 (95% CI 7-21) in the rifaximin-naïve group. Multivariate regression analysis demonstrated that rifaximin was independently associated with an increase in average days to readmission (adjusted effect estimate 71, 95% CI 3-140 days) and reduced likelihood of requirement for prioritisation on the waiting list (odds ratio 0.29; 95% CI 0.89-0.93).
Conclusion: Rifaximin prescribed for HE in patients listed for liver transplantation improved outcomes with significant reduction in admissions related to spontaneous bacterial peritonitis, ascites and variceal bleeding.
© 2019 The Authors. Alimentary Pharmacology & Therapeutics Published by John Wiley & Sons Ltd.
Figures
References
-
- Shawcross DL, Austin MJ, Abeles RD, et al. The impact of organ dysfunction in cirrhosis: survival at a cost? J Hepatol. 2012;56:1054‐1062. - PubMed
-
- Bustamante J, Rimola A, Ventura P‐J, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890‐895. - PubMed
-
- Cordoba J, Ventura‐Cots M, Simon‐Talero M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without ACLF (ACLF). J Hepatol. 2014;60:275‐281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
