Mitochondrial fusion is required for regulation of mitochondrial DNA replication
- PMID: 31170154
- PMCID: PMC6553695
- DOI: 10.1371/journal.pgen.1008085
Mitochondrial fusion is required for regulation of mitochondrial DNA replication
Abstract
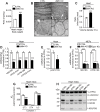
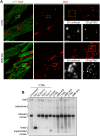
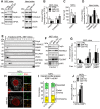
Mitochondrial dynamics is an essential physiological process controlling mitochondrial content mixing and mobility to ensure proper function and localization of mitochondria at intracellular sites of high-energy demand. Intriguingly, for yet unknown reasons, severe impairment of mitochondrial fusion drastically affects mtDNA copy number. To decipher the link between mitochondrial dynamics and mtDNA maintenance, we studied mouse embryonic fibroblasts (MEFs) and mouse cardiomyocytes with disruption of mitochondrial fusion. Super-resolution microscopy revealed that loss of outer mitochondrial membrane (OMM) fusion, but not inner mitochondrial membrane (IMM) fusion, leads to nucleoid clustering. Remarkably, fluorescence in situ hybridization (FISH), bromouridine labeling in MEFs and assessment of mitochondrial transcription in tissue homogenates revealed that abolished OMM fusion does not affect transcription. Furthermore, the profound mtDNA depletion in mouse hearts lacking OMM fusion is not caused by defective integrity or increased mutagenesis of mtDNA, but instead we show that mitochondrial fusion is necessary to maintain the stoichiometry of the protein components of the mtDNA replisome. OMM fusion is necessary for proliferating MEFs to recover from mtDNA depletion and for the marked increase of mtDNA copy number during postnatal heart development. Our findings thus link OMM fusion to replication and distribution of mtDNA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
Reshaping membranes to build mitochondrial DNA.PLoS Genet. 2019 Jun 6;15(6):e1008140. doi: 10.1371/journal.pgen.1008140. eCollection 2019 Jun. PLoS Genet. 2019. PMID: 31170157 Free PMC article. No abstract available.
References
-
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114: 867–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

