Different mechanisms of serum complement activation in the plasma of common (Chelydra serpentina) and alligator (Macrochelys temminckii) snapping turtles
- PMID: 31170203
- PMCID: PMC6553747
- DOI: 10.1371/journal.pone.0217626
Different mechanisms of serum complement activation in the plasma of common (Chelydra serpentina) and alligator (Macrochelys temminckii) snapping turtles
Erratum in
-
Correction: Different mechanisms of serum complement activation in the plasma of common (Chelydra serpentina) and alligator (Macrochelys temminckii) snapping turtles.PLoS One. 2019 Jul 10;14(7):e0219772. doi: 10.1371/journal.pone.0219772. eCollection 2019. PLoS One. 2019. PMID: 31291346 Free PMC article.
Abstract
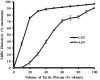
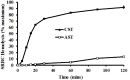
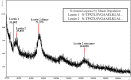
Reptiles are declining worldwide yet our understanding of their immune function lags far behind other taxa. The innate immune system is the primary mode of defense in reptiles, and the serum complement cascade is its major component. We assessed serum complement activity of plasma in two closely related aquatic turtle species, the common snapping turtle (CST; Chelydra serpentina) and alligator snapping turtle (AST; Macrochelys temminckii). We used a sheep red blood cell (SRBC) hemolysis assay to assess serum complement activity. Although the antibacterial activities of the plasma of these turtle species are similar, the hemolytic activity was much stronger in CST than AST. Treatment with inhibitors of the serum complement cascade indicated differences in the mechanisms of complement activation between the turtle species. We subjected plasma from both turtle species to mannan affinity chromatography and analyzed the eluate with SDS-PAGE, which revealed that plasma from the CSTs contained only small amounts of one C-type lectin protein while the AST plasma contained high concentrations of two C-type lectins (31.0 and 35.9 kDa). Edman degradation analyses confirmed that the two AST proteins contained identical N-terminal sequences. Thus, the CST appears to rely more heavily on the alternative mechanism of serum complement activation, while the AST appears to rely more on the lectin-mediated pathway, which is a pattern recognition response to prokaryotes not activated by the SRBCs. These results are unique in that the use of serum complement pathways are generally assumed to be conserved within clades.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

