Cost-effectiveness and budgetary impact of HCV treatment with direct-acting antivirals in India including the risk of reinfection
- PMID: 31170246
- PMCID: PMC6553784
- DOI: 10.1371/journal.pone.0217964
Cost-effectiveness and budgetary impact of HCV treatment with direct-acting antivirals in India including the risk of reinfection
Abstract
Background: HCV direct-acting antivirals (DAAs) are produced in India at low cost. However, concerns surrounding reinfection and budgetary impact limit treatment scale-up in India. We evaluate the cost-effectiveness and budgetary impact of HCV treatment in India, including reinfection.
Methods: A closed cohort Markov model of HCV disease progression, treatment, and reinfection was parameterized. We compared treatment by fibrosis stage (F2-F4 or F0-F4) to no treatment from a health care payer perspective. Costs (2017 USD$, based on India-specific data) and health utilities (in quality-adjusted life years, QALYs) were attached to each health state. We assumed DAAs with 90% sustained viral response at $900/treatment and 1%/year reinfection, varied in the sensitivity analysis from 0.1-15%. We deemed the intervention cost-effective if the incremental cost-effectiveness ratio (ICER) fell below India's per capita GDP ($1,709). We assessed the budgetary impact of treating all diagnosed individuals.
Results: HCV treatment for diagnosed F2-F4 individuals was cost-saving (net costs -$2,881 and net QALYs 3.18/person treated; negative ICER) compared to no treatment. HCV treatment remained cost-saving with reinfection rates of 15%/year. Treating all diagnosed individuals was likely cost-effective compared to delay until F2 (mean ICER $1,586/QALY gained, 67% of simulations falling under the $1,709 threshold) with 1%/year reinfection. For all scenarios, annual retesting for reinfection was more cost-effective than the current policy (one-time retest). Treating all diagnosed individuals and reinfections results in net costs of $445-1,334 million over 5 years (<0.25% of total health care expenditure over 5 years), and cost-savings within 14 years.
Conclusions: HCV treatment was highly cost-effective in India, despite reinfection. Annual retesting for reinfection was cost-effective, supporting a policy change towards more frequent retesting. A comprehensive HCV treatment scale-up plan is warranted in India.
Conflict of interest statement
NM has received unrestricted research grants and honoraria from Gilead and Merck. PV has received unrestricted research grants from Gilead and honoraria from Gilead and Abbvie. MHo has received research grants from Gilead. MHi has received honoraria from Gilead, MSD and Abbvie. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


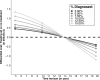
References
-
- Observatory P. Hepatitis C Report 2017 [updated 07/26/17; cited 2017 08/20/17]. Available from: http://polarisobservatory.org/polaris/hepC.htm.
-
- (WHO) WHO. Global hepatitis report. 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

