Redox properties and human serum albumin binding of nitro-oleic acid
- PMID: 31170679
- PMCID: PMC6554544
- DOI: 10.1016/j.redox.2019.101213
Redox properties and human serum albumin binding of nitro-oleic acid
Abstract
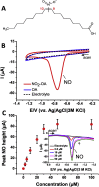
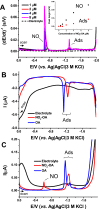
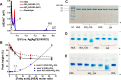
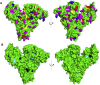
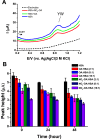
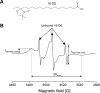
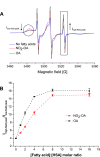
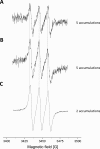
Nitro-fatty acids modulate inflammatory and metabolic stress responses, thus displaying potential as new drug candidates. Herein, we evaluate the redox behavior of nitro-oleic acid (NO2-OA) and its ability to bind to the fatty acid transporter human serum albumin (HSA). The nitro group of NO2-OA underwent electrochemical reduction at -0.75 V at pH 7.4 in an aqueous milieu. Based on observations of the R-NO2 reduction process, the stability and reactivity of NO2-OA was measured in comparison to oleic acid (OA) as the negative control. These electrochemically-based results were reinforced by computational quantum mechanical modeling. DFT calculations indicated that both the C9-NO2 and C10-NO2 positional isomers of NO2-OA occurred in two conformers with different internal angles (69° and 110°) between the methyl- and carboxylate termini. Both NO2-OA positional isomers have LUMO energies of around -0.7 eV, affirming the electrophilic properties of fatty acid nitroalkenes. In addition, the binding of NO2-OA and OA with HSA revealed a molar ratio of ~7:1 [NO2-OA]:[HSA]. These binding experiments were performed using both an electrocatalytic approach and electron paramagnetic resonance (EPR) spectroscopy using 16-doxyl stearic acid. Using a Fe(DTCS)2 spin-trap, EPR studies also showed that the release of the nitro moiety of NO2-OA resulted in the formation of nitric oxide radical. Finally, the interaction of NO2-OA with HSA was monitored via Tyr and Trp residue electro-oxidation. The results indicate that not only non-covalent binding but also NO2-OA-HSA adduction mechanisms should be taken into consideration. This study of the redox properties of NO2-OA is applicable to the characterization of other electrophilic mediators of biological and pharmacological relevance.
Keywords: Electrophiles; NO; Nitrated fatty acids; Oleic acid; Proteins; Serum albumin binding.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Tornqvist M., Fred C., Haglund J., Helleberg H., Paulsson B., Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B. 2002;778:279–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

