Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry
- PMID: 31171024
- PMCID: PMC6555030
- DOI: 10.1186/s13075-019-1917-8
Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry
Abstract
Background: Treatment persistence is an important consideration when selecting a therapy for chronic conditions such as rheumatoid arthritis (RA). We assessed the long-term persistence of abatacept or a tumor necrosis factor inhibitor (TNFi) following (1) inadequate response to a conventional synthetic disease-modifying antirheumatic drug (first-line biologic agent) and (2) inadequate response to a first biologic DMARD (second-line biologic agent).
Methods: Data were extracted from the Rhumadata® registry for patients with RA prescribed either abatacept or a TNFi (adalimumab, certolizumab, etanercept, golimumab, or infliximab) who met the study selection criteria. The primary outcome was persistence to abatacept and TNFi treatment, as first- or second-line biologics. Secondary outcomes included the proportion of patients discontinuing therapy, reasons for discontinuation, and predictors of discontinuation. Persistence was defined as the time from initiation to discontinuation of biologic therapy. Baseline characteristics were compared using descriptive statistics; cumulative persistence rates were estimated using Kaplan-Meier methods, compared using the log-rank test. Multivariate Cox proportional hazard models were used to compare the persistence between treatments, controlling for baseline covariates.
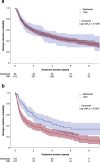
Results: Overall, 705 patients met the selection criteria for first-line biologic agent initiation (abatacept, n = 92; TNFi, n = 613) and 317 patients met the criteria for second-line biologic agent initiation (abatacept, n = 105; TNFi, n = 212). There were no clinically significant differences in baseline characteristics between the treatments with either first- or second-line biologics. Persistence was similar between the first-line biologic treatments (p = 0.7406) but significantly higher for abatacept compared with TNFi as a second-line biologic (p = 0.0001). Mean (SD) times on first-line biologic abatacept and TNFi use were 4.53 (0.41) and 5.35 (0.20) years, and 4.80 (0.45) and 2.82 (0.24) years, respectively, as second-line biologic agents. The proportion of patients discontinuing abatacept and TNFi in first-line was 51.1% vs. 59.5% (p = 0.1404), respectively. In second-line, it was 57.1% vs. 74.1% (p = 0.0031). The main reasons for stopping both treatments were inefficacy and adverse events.
Conclusions: Abatacept and TNFi use demonstrated similar persistence rates at 9 years as a first-line biologic agent. As a second-line biologic agent, abatacept had better persistence rates over a TNFi.
Keywords: Abatacept; Disease-modifying antirheumatic drugs (biologic); Persistence; Registry; Rheumatoid arthritis; TNF inhibitor.
Conflict of interest statement
DC is a consultant and speaker for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Merck, Novartis, Pfizer, Roche, and Sandoz. LB is a consultant and speaker for, and has received research support from, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer, Roche, and UCB; in addition, LB has received speaker fees from Merck and research support from Merck and Sanofi. EA and RP are employees of and/or shareholders of and/or hold stock options in Bristol-Myers Squibb. BH is a consultant for AbbVie, Amgen, Lilly, Merck, Pfizer, and UCB; in addition, BH is a speaker for Pfizer and has received research support from AbbVie. J-PR is a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB; in addition, J-PR has received consulting fees and research support from Arthrovision Inc. LC declares no competing interests.
Figures

References
-
- Bykerk VP, Akhavan P, Hazlewood GS, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association Recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;39(8):1559–1582. doi: 10.3899/jrheum.110207. - DOI - PubMed
-
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. - DOI - PubMed
-
- Régie de l’assurance maladie Québec. Liste des médicaments – Établissements: Dépôt légal — Bibliothèque et Archives nationales du Québec; 2018 [updated 24 mai 2018. Available from: http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/liste_med/2019/liste_.... Accessed 14 Aug 2018.
-
- Codullo V, Iannone F, Sinigaglia L, Favalli EG, Sarzi-Puttini P, Atzeni F, et al. Comparison of efficacy of first- versus second-line adalimumab in patients with rheumatoid arthritis: experience of the Italian biologics registries. Clin Exp Rheumatol. 2017;35(4):660–665. - PubMed
-
- Gottenberg JE, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA. 2016;316(11):1172–1180. doi: 10.1001/jama.2016.13512. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

