Temporal Modulation of HER2 Membrane Availability Increases Pertuzumab Uptake and Pretargeted Molecular Imaging of Gastric Tumors
- PMID: 31171598
- PMCID: PMC6836866
- DOI: 10.2967/jnumed.119.225813
Temporal Modulation of HER2 Membrane Availability Increases Pertuzumab Uptake and Pretargeted Molecular Imaging of Gastric Tumors
Abstract
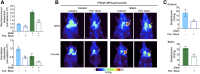
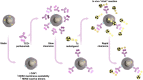
Human epidermal growth factor receptor 2 (HER2) is used as a tumor biomarker and therapeutic target. Pertuzumab is an anti-HER2 antibody, and its binding to tumor cells requires HER2 to be present at the cell membrane. However, the cellular distribution of HER2 protein in gastric tumors is dynamic, and HER2 internalization decreases antibody binding to tumor cells. These features preclude the use of pretargeted strategies for molecular imaging and therapy. We explored the pharmacological modulation of HER2 endocytosis as a strategy to improve pertuzumab uptake in HER2-positive gastric tumors and allow the use of a pretargeted imaging approach. Methods: We conducted in vitro and in vivo studies with NCI-N87 gastric cancer cells to determine how HER2 endocytosis affects pertuzumab binding to tumor cells. Lovastatin, a clinically approved cholesterol-lowering drug, was used to modulate caveolae-mediated HER2 endocytosis. Results: Administration of lovastatin to NCI-N87 cancer cells resulted in significant accumulation of non-activated HER2 dimers at the cell surface. Pretreatment of NCI-N87 cells with lovastatin increased in vitro specific accumulation of membrane-bound 89Zr-labeled pertuzumab. Lovastatin-enhanced pertuzumab tumor uptake was also observed in NCI-N87 gastric cancer xenografts, allowing tumor detection as early as 4 h and high-contrast images at 48 h after tracer administration via PET. Temporal enhancement of HER2 membrane availability by lovastatin allowed imaging of cell surface HER2 with transcyclooctene-conjugated antibodies and 18F-labeled tetrazine. Conclusion: Temporal pharmacological modulation of membrane HER2 may be clinically relevant and exploitable for pretargeted molecular imaging and therapy in gastric tumors.
Keywords: HER2; gastric tumors; lovastatin; pertuzumab; pretargeting.
© 2019 by the Society of Nuclear Medicine and Molecular Imaging.
Figures




References
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst. 2013;105:266–273. - PubMed
-
- Hedner C, Borg D, Nodin B, Karnevi E, Jirstrom K, Eberhard J. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in oesophageal and gastric adenocarcinomas preneoadjuvant and postneoadjuvant treatment. J Clin Pathol. 2018;71:451–462. - PubMed
-
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
