Age at onset in genetic prion disease and the design of preventive clinical trials
- PMID: 31171647
- PMCID: PMC6656649
- DOI: 10.1212/WNL.0000000000007745
Age at onset in genetic prion disease and the design of preventive clinical trials
Abstract
Objective: To determine whether preventive trials in genetic prion disease could be designed to follow presymptomatic mutation carriers to onset of disease.
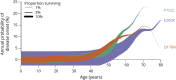
Methods: We assembled age at onset or death data from 1,094 individuals with high penetrance mutations in the prion protein gene (PRNP) in order to generate survival and hazard curves and test for genetic modifiers of age at onset. We used formulae and simulations to estimate statistical power for clinical trials.
Results: Genetic prion disease age at onset varies over several decades for the most common mutations and neither sex, parent's age at onset, nor PRNP codon 129 genotype provided additional explanatory power to stratify trials. Randomized preventive trials would require hundreds or thousands of at-risk individuals in order to be statistically powered for an endpoint of clinical onset, posing prohibitive cost and delay and likely exceeding the number of individuals available for such trials.
Conclusion: The characterization of biomarkers suitable to serve as surrogate endpoints will be essential for the prevention of genetic prion disease. Parameters such as longer trial duration, increased enrollment, and the use of historical controls in a postmarketing study could provide opportunities for subsequent determination of clinical benefit.
© 2019 American Academy of Neurology.
Figures
References
-
- New drug, antibiotic, and biological drug product regulations; accelerated approval: FDA: final rule. Fed Regist 1992;57:58942–58960. - PubMed
-
- Food and Drug Administration Safety and Innovation Act. Public Law 112-114 Section 506(c)(3). July 9, 2012.
-
- Otto M, Cepek L, Ratzka P, et al. . Efficacy of flupirtine on cognitive function in patients with CJD: a double-blind study. Neurology 2004;62:714–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
