Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells
- PMID: 31171772
- PMCID: PMC6554411
- DOI: 10.1038/s41467-019-10189-x
Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells
Abstract
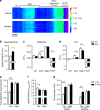
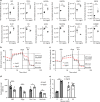
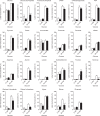
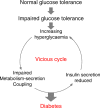
Diabetes is a global health problem caused primarily by the inability of pancreatic β-cells to secrete adequate levels of insulin. The molecular mechanisms underlying the progressive failure of β-cells to respond to glucose in type-2 diabetes remain unresolved. Using a combination of transcriptomics and proteomics, we find significant dysregulation of major metabolic pathways in islets of diabetic βV59M mice, a non-obese, eulipidaemic diabetes model. Multiple genes/proteins involved in glycolysis/gluconeogenesis are upregulated, whereas those involved in oxidative phosphorylation are downregulated. In isolated islets, glucose-induced increases in NADH and ATP are impaired and both oxidative and glycolytic glucose metabolism are reduced. INS-1 β-cells cultured chronically at high glucose show similar changes in protein expression and reduced glucose-stimulated oxygen consumption: targeted metabolomics reveals impaired metabolism. These data indicate hyperglycaemia induces metabolic changes in β-cells that markedly reduce mitochondrial metabolism and ATP synthesis. We propose this underlies the progressive failure of β-cells in diabetes.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- UKPDS. UK. Prospective diabetes study 16: overview of 6 years’ therapy of type ii diabetes: a progressive disease. Diabetes. 1998;44:1249–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

