Combined Endoscopic and Oral Fecal Microbiota Transplantation in Patients with Antibiotic-Dependent Pouchitis: Low Clinical Efficacy due to Low Donor Microbial Engraftment
- PMID: 31172007
- PMCID: PMC6537468
- DOI: 10.1159/000497042
Combined Endoscopic and Oral Fecal Microbiota Transplantation in Patients with Antibiotic-Dependent Pouchitis: Low Clinical Efficacy due to Low Donor Microbial Engraftment
Abstract
Background and objective: A significant number of pouch patients develop antibiotic-dependent pouchitis (ADP). Microbial dysbiosis is thought to be a major driver of clinical symptoms in ADP. The objective of this proof of concept study was to evaluate safety, efficacy, and donor microbial engraftment of an intensified fecal microbiota transplant (FMT) consisting of a single endoscopic FMT followed by daily oral FMT for 2 weeks in patients with ADP.
Methods: We performed a prospective placebo-controlled double-blind FMT trial in patents with established ADP and planned to enroll 20 patients in this proof of concept study. In case of non-response, patients were offered an optional open label active FMT treatment. The endpoints were safety, clinical remission without need for antibiotics during 16 weeks of follow-up, quantitative changes of fecal calprotectin (FCP), and engraftment of donor FMT as determined by metagenomic sequencing of the V4 region of the 16S rRNA gene.
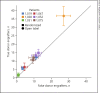
Results: Due to a lower than expected clinical remission rate and low FMT engraftment, enrollment in the study was stopped prematurely after 6 patients were included. All 6 patients enrolled in the placebo-controlled portion failed to respond and needed antibiotic rescue therapy shortly after FMT. FCP increased in the majority of patients in the setting of relapse after FMT. In the active open label FMT extension study 1 out of 5 patients achieved antibiotic-free clinical remission. FMT engraftment after active FMT was observed only in this single patient, whereas engraftment of donor FMT occurred in none of the other patients receiving active FMT, paralleling the lack of clinical response.
Conclusions: Low donor FMT engraftment resulted in low clinical efficacy of FMT in patients with ADP. Before embarking on larger clinical trials with FMT in patients with ADP or other forms of pouchitis, it is mandatory to explore approaches for superior FMT engraftment.
Keywords: Fecal microbiota transplantation; Inflammatory bowel diseases; Microbiome; Pouchitis; Stool transplant.
Figures
References
-
- Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EV, Jr, Epidemiology and Natural History Task Force of the International Organization of the Study of Inflammatory Bowel Disease A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013 Aug;19((9)):2001–2010. - PubMed
-
- Shen B, Fazio VW, Remzi FH, Lashner BA. Clinical approach to diseases of ileal pouch-anal anastomosis. Am J Gastroenterol. 2005 Dec;100((12)):2796–807. - PubMed
-
- Segal JP, Ding NS, Worley G, Mclaughlin S, Preston S, Faiz OD, et al. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther. 2017 Mar;45((5)):581–92. - PubMed
-
- Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013 Apr;108((4)):500–8. - PubMed
LinkOut - more resources
Full Text Sources

