Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer
- PMID: 31172284
- PMCID: PMC6954126
- DOI: 10.1007/s00520-019-04875-1
Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer
Abstract
Purpose: The effects of chemotherapy dose intensity on survival in patients with advanced non-small-cell lung cancer (NSCLC) are poorly understood. We retrospectively analyzed dose delays/reduction, relative dose intensity (RDI), and the association between chemotherapy intensity and survival in advanced NSCLC.
Methods: This retrospective cohort study included adults with advanced lung cancer who received first-line myelosuppressive platinum-based chemotherapy (January 2007-December 2010) in ~ 230 US Oncology Network community practices. Dose delays ≥ 7 days, dose reductions ≥ 15%, and RDI relative to standard regimens were described. Overall survival (OS) was measured using Kaplan-Meier and Cox proportional hazard (PH) models.
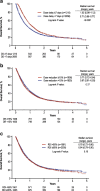
Results: Among 3866 patients with advanced NSCLC, 32.4% experienced dose delays ≥ 7 days, 50.1% experienced dose reductions ≥ 15%, and 40.4% had RDI < 85%. Reduced RDI was also common regardless of baseline ECOG PS (ECOG PS ≥ 2, 56.2%; ECOG PS 0, 33.6%) and tumor subgroup (squamous cell carcinoma, 52.2%; adenocarcinoma, 36.0%). When stratified by chemotherapy intensity measures, significant OS differences were observed only for dose delays. Median (95% CI) OS was 1.02 years (0.96-1.12) for dose delays ≥ 7 days and 0.71 years (0.66-0.77) for dose delays < 7 days. In multivariable Cox PH analysis, dose delays ≥ 7 days (HR = 0.71; 95% CI = 0.63-0.80) and RDI ≥ 85% (HR = 1.18; 95% CI = 1.05-1.32) were significantly associated with decreased mortality.
Conclusions: Dose delays, dose reductions, and reduced RDI were common, and dose delays ≥ 7 days and high RDI were significantly associated with decreased mortality. These results can help identify potential risk factors and characterize the effect of chemotherapy dose modification strategies on mortality.
Keywords: Chemotherapy; Community health services; Lung cancer; Retrospective studies.
Conflict of interest statement
McKesson Specialty Health received research funding from Amgen Inc. to complete this work. JC and GHL are principal investigators on research grants from Amgen Inc. for their respective institutions. ND and XJ are or were employees of McKesson Specialty Health. PKM, JG, and RB are or were employees of and own stock in Amgen Inc. DP has nothing to disclose.
There is a plan to share data. This may include de-identified individual patient data for variables necessary to address the specific research question in an approved data-sharing request, also related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report. Data-sharing requests relating to data in this manuscript will be considered after the publication date and (1) this product and indication (or other new use) have been granted marketing authorization in both the USA and Europe, or (2) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data-sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, endpoints/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researcher(s). In general, Amgen does not grant external requests for individual patient data for the purpose of re-evaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors reviews requests. If not approved, requests may be further arbitrated by a Data Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen’s sole discretion and without further arbitration. Upon approval, information necessary to address the research question will be provided under the terms of a data-sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications. Further details are available at the following:
Figures
References
-
- National Cancer Institute (2017) Statistics at a glance: lung and bronchus. Available at: https://seer.cancer.gov/statfacts/html/lungb.html. Accessed May 11, 2017
-
- National Comprehensive Cancer Network (2017) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-small cell lung cancer, version 4.2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed January 23, 2017 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical