Reduced Exposure to Piperaquine, Compared to Adults, in Young Children Receiving Dihydroartemisinin-Piperaquine as Malaria Chemoprevention
- PMID: 31173649
- PMCID: PMC6851416
- DOI: 10.1002/cpt.1534
Reduced Exposure to Piperaquine, Compared to Adults, in Young Children Receiving Dihydroartemisinin-Piperaquine as Malaria Chemoprevention
Abstract
Dihydroartemisinin (DHA)-piperaquine is being evaluated as intermittent preventive therapy for malaria, but dosing has not been optimized for children. We assessed exposure to DHA and piperaquine in Ugandan children at two ages during infancy. Intensive sampling was performed in 32 children at 32 weeks of age, 31 children at 104 weeks, and 30 female adult controls. Compared with adults, DHA area under the concentration-time curve (AUC0-8 hr ) was 52% higher at 32 weeks and comparable at 104 weeks. Compared with adults, piperaquine AUC0-21 d was 35% lower at 32 weeks and 53% lower at 104 weeks. Terminal piperaquine concentrations on days 7, 14, and 21 were lower in children compared with adults and lower at 104 compared with 32 weeks. Piperaquine exposure was lower in young children compared with adults, and lower at 104 compared with 32 weeks of age, suggesting a need for age-based DHA-piperaquine dose optimization for chemoprevention.
Trial registration: ClinicalTrials.gov NCT02163447.
© 2019 The Authors Clinical Pharmacology & Therapeutics © 2019 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Figures


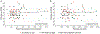
References
-
- World Health Organization. (2018). World Malaria Report 2018 (https://www.who.int/malaria/media/world-malaria-report-2018/en/, 2018).
-
- Walker PG, ter Kuile FO, Garske T, Menendez C & Ghani AC Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health 2, e460–7 (2014). - PubMed
-
- Steketee RW, Nahlen BL, Parise ME & Menendez C The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64, 28–35 (2001). - PubMed
-
- World Health Organization. Intermittent preventive treatment in pregnancy (IPTp). <https://www.who.int/malaria/areas/preventive_therapies/pregnancy/en/> (2018). Accessed January 31 2019.
-
- World Health Organization. Seasonal malaria chemoprevention (SMC). <https://www.who.int/malaria/areas/preventive_therapies/children/en/> (2017). Accessed January 31 2019.

