Mitotic motor CENP-E cooperates with PRC1 in temporal control of central spindle assembly
- PMID: 31174204
- PMCID: PMC7683015
- DOI: 10.1093/jmcb/mjz051
Mitotic motor CENP-E cooperates with PRC1 in temporal control of central spindle assembly
Abstract
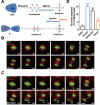
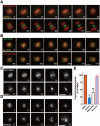
Error-free cell division depends on the accurate assembly of the spindle midzone from dynamic spindle microtubules to ensure chromatid segregation during metaphase-anaphase transition. However, the mechanism underlying the key transition from the mitotic spindle to central spindle before anaphase onset remains elusive. Given the prevalence of chromosome instability phenotype in gastric tumorigenesis, we developed a strategy to model context-dependent cell division using a combination of light sheet microscope and 3D gastric organoids. Light sheet microscopic image analyses of 3D organoids showed that CENP-E inhibited cells undergoing aberrant metaphase-anaphase transition and exhibiting chromosome segregation errors during mitosis. High-resolution real-time imaging analyses of 2D cell culture revealed that CENP-E inhibited cells undergoing central spindle splitting and chromosome instability phenotype. Using biotinylated syntelin as an affinity matrix, we found that CENP-E forms a complex with PRC1 in mitotic cells. Chemical inhibition of CENP-E in metaphase by syntelin prevented accurate central spindle assembly by perturbing temporal assembly of PRC1 to the midzone. Thus, CENP-E-mediated PRC1 assembly to the central spindle constitutes a temporal switch to organize dynamic kinetochore microtubules into stable midzone arrays. These findings reveal a previously uncharacterized role of CENP-E in temporal control of central spindle assembly. Since CENP-E is absent from yeast, we reasoned that metazoans evolved an elaborate central spindle organization machinery to ensure accurate sister chromatid segregation during anaphase and cytokinesis.
Keywords: CENP-E; PRC1; cell division; central spindle; organoids; syntelin.
© The Author(s) (2019). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS.
Figures



References
-
- Akhmanova, A., and Steinmetz, M.O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309–322. - PubMed

