Online yoga in myeloproliferative neoplasm patients: results of a randomized pilot trial to inform future research
- PMID: 31174535
- PMCID: PMC6556039
- DOI: 10.1186/s12906-019-2530-8
Online yoga in myeloproliferative neoplasm patients: results of a randomized pilot trial to inform future research
Abstract
Background: Myeloproliferative neoplasm (MPN) patients suffer from significant symptoms, inflammation and reduced quality of life. Yoga improves these outcomes in other cancers, but this hasn't been demonstrated in MPNs. The purpose of this study was to: (1) explore the limited efficacy (does the program show promise of success) of a 12-week online yoga intervention among MPN patients on symptom burden and quality of life and (2) determine feasibility (practicality: to what extent a measure can be carried out) of remotely collecting inflammatory biomarkers.
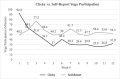
Methods: Patients were recruited nationally and randomized to online yoga (60 min/week of yoga) or wait-list control (asked to maintain normal activity). Weekly yoga minutes were collected with Clicky (online web analytics tool) and self-report. Those in online yoga completed a blood draw at baseline and week 12 to assess inflammation (interleukin-6, tumor necrosis factor-alpha [TNF-α]). All participants completed questionnaires assessing depression, anxiety, fatigue, pain, sleep disturbance, sexual function, total symptom burden, global health, and quality of life at baseline, week seven, 12, and 16. Change from baseline at each time point was computed by group and effect sizes were calculated. Pre-post intervention change in inflammation for the yoga group was compared by t-test.
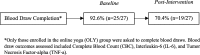
Results: Sixty-two MPN patients enrolled and 48 completed the intervention (online yoga = 27; control group = 21). Yoga participation averaged 40.8 min/week via Clicky and 56.1 min/week via self-report. Small/moderate effect sizes were generated from the yoga intervention for sleep disturbance (d = - 0.26 to - 0.61), pain intensity (d = - 0.34 to - 0.51), anxiety (d = - 0.27 to - 0.37), and depression (d = - 0.53 to - 0.78). A total of 92.6 and 70.4% of online yoga participants completed the blood draw at baseline and week 12, respectively, and there was a decrease in TNF-α from baseline to week 12 (- 1.3 ± 1.5 pg/ml).
Conclusions: Online yoga demonstrated small effects on sleep, pain, and anxiety as well as a moderate effect on depression. Remote blood draw procedures are feasible and the effect size of the intervention on TNF-α was large. Future fully powered randomized controlled trials are needed to test for efficacy.
Trial registration: This trial was retrospectively registered with clinicaltrials.gov (ID: NCT03503838 ) on 4/19/2018.
Keywords: Cancer; Complementary; Mindfulness; Neoplasm; Physical activity.
Conflict of interest statement
The authors that they have no competing interests.
Figures
References
-
- Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103. doi: 10.1200/JCO.2012.42.3863. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical