The tree shrew is a promising model for the study of influenza B virus infection
- PMID: 31174549
- PMCID: PMC6555921
- DOI: 10.1186/s12985-019-1171-3
The tree shrew is a promising model for the study of influenza B virus infection
Abstract
Background: Influenza B virus is a main causative pathogen of annual influenza epidemics, however, research on influenza B virus in general lags behind that on influenza A viruses, one of the important reasons is studies on influenza B viruses in animal models are limited. Here we investigated the tree shrew as a potential model for influenza B virus studies.
Methods: Tree shrews and ferrets were inoculated with either a Yamagata or Victoria lineage influenza B virus. Symptoms including nasal discharge and weight loss were observed. Nasal wash and respiratory tissues were collected at 2, 4 and 6 days post inoculation (DPI). Viral titers were measured in nasal washes and tissues were used for pathological examination and extraction of mRNA for measurement of cytokine expression.
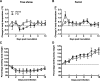
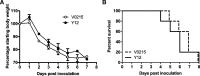
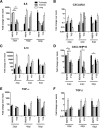
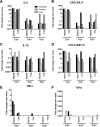
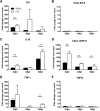
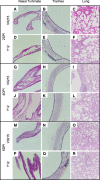
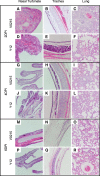
Results: Clinical signs and pathological changes were also evident in the respiratory tracts of tree shrews and ferrets. Although nasal symptoms including sneezing and rhinorrhea were evident in ferrets infected with influenza B virus, tree shrews showed no significant respiratory symptoms, only milder nasal secretions appeared. Weight loss was observed in tree shrews but not ferrets. V0215 and Y12 replicated in all three animal (ferrets, tree shrews and mice) models with peak titers evident on 2DPI. There were no significant differences in peak viral titers in ferrets and tree shrews inoculated with Y12 at 2 and 4DPI, but viral titers were detected at 6DPI in tree shrews. Tree shrews infected with influenza B virus showed similar seroconversion and respiratory tract pathology to ferrets. Elevated levels of cytokines were detected in the tissues isolated from the respiratory tract after infection with either V0215 or Y12 compared to the levels in the uninfected control in both animals. Overall, the tree shrew was sensitive to infection and disease by influenza B virus.
Conclusion: The tree shrew to be a promising model for influenza B virus research.
Keywords: Animal model; Ferret; Influenza B virus; List of Abbreviations.; Mouse; Tree shrew.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Tree Shrew as an Emerging Small Animal Model for Human Viral Infection: A Recent Overview.Viruses. 2021 Aug 18;13(8):1641. doi: 10.3390/v13081641. Viruses. 2021. PMID: 34452505 Free PMC article. Review.
-
Study of tree shrew biology and models: A booming and prosperous field for biomedical research.Zool Res. 2024 Jul 18;45(4):877-909. doi: 10.24272/j.issn.2095-8137.2024.199. Zool Res. 2024. PMID: 39004865 Free PMC article. Review.
-
Characterization of the Localized Immune Response in the Respiratory Tract of Ferrets following Infection with Influenza A and B Viruses.J Virol. 2015 Dec 30;90(6):2838-48. doi: 10.1128/JVI.02797-15. J Virol. 2015. PMID: 26719259 Free PMC article.
-
The tree shrew provides a useful alternative model for the study of influenza H1N1 virus.Virol J. 2013 Apr 10;10:111. doi: 10.1186/1743-422X-10-111. Virol J. 2013. PMID: 23575279 Free PMC article.
-
Herpes Simplex Virus 1 Infection of Tree Shrews Differs from That of Mice in the Severity of Acute Infection and Viral Transcription in the Peripheral Nervous System.J Virol. 2015 Oct 28;90(2):790-804. doi: 10.1128/JVI.02258-15. Print 2016 Jan 15. J Virol. 2015. PMID: 26512084 Free PMC article.
Cited by
-
Tree Shrew as an Emerging Small Animal Model for Human Viral Infection: A Recent Overview.Viruses. 2021 Aug 18;13(8):1641. doi: 10.3390/v13081641. Viruses. 2021. PMID: 34452505 Free PMC article. Review.
-
Study of tree shrew biology and models: A booming and prosperous field for biomedical research.Zool Res. 2024 Jul 18;45(4):877-909. doi: 10.24272/j.issn.2095-8137.2024.199. Zool Res. 2024. PMID: 39004865 Free PMC article. Review.
-
Comparative Pathogenicity and Transmissibility of Pandemic H1N1, Avian H5N1, and Human H7N9 Influenza Viruses in Tree Shrews.Front Microbiol. 2019 Dec 20;10:2955. doi: 10.3389/fmicb.2019.02955. eCollection 2019. Front Microbiol. 2019. PMID: 31921093 Free PMC article.
-
Host Diversity and Potential Transmission Pathways of SARS-CoV-2 at the Human-Animal Interface.Pathogens. 2021 Feb 8;10(2):180. doi: 10.3390/pathogens10020180. Pathogens. 2021. PMID: 33567598 Free PMC article. Review.
-
Identification and characterization of the tumor necrosis factor receptor superfamily in the Chinese tree shrew (Tupaia belangeri chinensis).BMC Genomics. 2025 Apr 4;26(1):338. doi: 10.1186/s12864-025-11451-x. BMC Genomics. 2025. PMID: 40186114 Free PMC article.
References
-
- [Database] World Health Organization. Global influenza surveillance and response system (GISRS). 2018. http://www.who.int/influenza/gisrs_laboratory/en/. 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

