Nek2B activates the wnt pathway and promotes triple-negative breast cancer chemothezrapy-resistance by stabilizing β-catenin
- PMID: 31174562
- PMCID: PMC6556028
- DOI: 10.1186/s13046-019-1231-y
Nek2B activates the wnt pathway and promotes triple-negative breast cancer chemothezrapy-resistance by stabilizing β-catenin
Abstract
Background: The chemotherapy-resistance of triple-negative breast cancer (TNBC) remains a major challenge. The Nek2B kinase and β-catenin serve as crucial regulators of mitotic processes. The aim of this study was to test the correlation between Nek2B and TNBC chemotherapy sensitivity, and to determine the regulation of Nek2B on β-catenin and wnt/β-catenin signal pathway.
Methods: Gene Expression Omnibus(GEO) databases were used to gather gene exprsssion data of TNBC patients who undergoing chemotherapy. The co-expression of Nek2B and β-catenin in TNBC surgical sections and cells were analysed by immunohistochemistry, Q-RT-PCR, Western-blot and immunofluorescent staining. The impact of the expression of Nek2B and β-catenin in prognosis was also assessed using the Kaplan-Meier curves. CCK8 assay was used to detect the IC50 value of TNBC cell line. The endogenous binding capacity of Nek2B and β-catenin and phosphorylation of β-catenin by Nek2B were detected using co-immunoprecipitation (CO-IP). Chromatin immune-precipitation (ChIP) analysis and Luciferase Assays were used to evaluate the binding ability of the Nek2B, β-catenin and TCF4 complex with LEF-1 promoter. Nek2B-siRNA and Nek2B plasmid were injected into nude mice, and tumorigenesis was monitored.
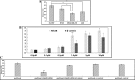
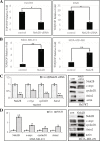
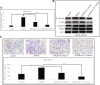
Results: We found that overexpression of Nek2B and β-catenin in TNBC samples, was associated with patients poor prognosis. Patients with positive Nek2B expression were less sensitive to paclitaxel-containing neoadjuvant chemotherapy. Interestingly, in a panel of established TNBC cell line, Nek2B and β-catenin were highly expressed in cells exhibiting paclitaxel resistance. Our data also suggest that β-catenin binded to and was phosphorylated by Nek2B, and was in a complex with TCF4. Nek2B mainly regulates the expression of β-catenin in TNBC nucleus. Nek2B, β-catenin and TCF4 can be binded with the WRE functional area of LEF-1 promoter. Nek2B can activite wnt signaling pathway and wnt downstream target genes. The tumors treated by Nek2B siRNA associated with paclitaxel were the smallest in nude mouse, and Nek2B can regulate the expression of β-catenin and wnt downstream target genes in vivo.
Conclusion: Our study suggested that Nek2B can bind to β-catenin and the co-expression correlated with TNBC patients poor prognosis. It appears that Nek2B and β-catenin might synergize to promote chemotherapy resistance.
Keywords: Chemotherapy resistance; Nek2B; Triple-negative breast cancer; Wnt signaling pathway; β-Catenin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures











References
-
- Su Y, Hopfinger NR, Nguyen TD, Pogash TJ, Santucci-Pereira J, Russo J. Epigenetic reprogramming of epithelial mesenchymal transition in triple negative breast cancercells with DNA methyltransferase and histone deacetylase inhibitors. J Exp Clin Cancer Res. 2018;37:314. doi: 10.1186/s13046-018-0988-8. - DOI - PMC - PubMed
-
- Logue SE, McGrath EP, Cleary P, Greene S, Mnich K, Almanza A, Chevet E, Dwyer RM, Oommen A, Legembre P, Godey F, Madden EC, Leuzzi B, Obacz J, Zeng Q, Patterson JB, Jäger R, Gorman AM, Samali A. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat Commun. 2018;9:3267. doi: 10.1038/s41467-018-05763-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

