Resistance and resilience to Alzheimer's disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort
- PMID: 31174609
- PMCID: PMC6556006
- DOI: 10.1186/s40478-019-0743-1
Resistance and resilience to Alzheimer's disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort
Erratum in
-
Publisher Correction to: Acta Neuropathologica Communications, volume 7.Acta Neuropathol Commun. 2019 Aug 14;7(1):131. doi: 10.1186/s40478-019-0784-5. Acta Neuropathol Commun. 2019. PMID: 31412936 Free PMC article.
Abstract
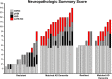
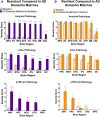
Alzheimer's disease neuropathologic change (ADNC) is defined by progressive accumulation of β-amyloid plaques and hyperphosphorylated tau (pTau) neurofibrillary tangles across diverse regions of brain. Non-demented individuals who reach advanced age without significant ADNC are considered to be resistant to AD, while those burdened with ADNC are considered to be resilient. Understanding mechanisms underlying ADNC resistance and resilience may provide important clues to treating and/or preventing AD associated dementia. ADNC criteria for resistance and resilience are not well-defined, so we developed stringent pathologic cutoffs for non-demented subjects to eliminate cases of borderline pathology. We identified 14 resistant (85+ years old, non-demented, Braak stage ≤ III, CERAD absent) and 7 resilient (non-demented, Braak stage VI, CERAD frequent) individuals out of 684 autopsies from the Adult Changes in Thought study, a long-standing community-based cohort. We matched each resistant or resilient subject to a subject with dementia and severe ADNC (Braak stage VI, CERAD frequent) by age, sex, year of death, and post-mortem interval. We expanded the neuropathologic evaluation to include quantitative approaches to assess neuropathology and found that resilient participants had lower neocortical pTau burden despite fulfilling criteria for Braak stage VI. Moreover, limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) was robustly associated with clinical dementia and was more prevalent in cases with high pTau burden, supporting the notion that resilience to ADNC may depend, in part, on resistance to pTDP-43 pathology. To probe for interactions between tau and TDP-43, we developed a C. elegans model of combined human (h) Tau and TDP-43 proteotoxicity, which exhibited a severe degenerative phenotype most compatible with a synergistic, rather than simply additive, interaction between hTau and hTDP-43 neurodegeneration. Pathways that underlie this synergy may present novel therapeutic targets for the prevention and treatment of AD.
Keywords: Alzheimer’s disease neuropathologic change; C. elegans; Dementia; Hyperphosphorylated tau; Resilience; Resistance; TDP-43.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- American Psychiatric Association. Task force on DSM-IV. (2000) Diagnostic and statistical manual of mental disorders: DSM-II. Am Psychiatr Publ. doi: http://dx.doi.org.lrc1.usuhs.edu/10.1176/appi.books.9780890425596 - DOI
-
- Aoki Naoya, Murray Melissa E., Ogaki Kotaro, Fujioka Shinsuke, Rutherford Nicola J., Rademakers Rosa, Ross Owen A., Dickson Dennis W. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathologica. 2014;129(1):53–64. doi: 10.1007/s00401-014-1358-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA015704/CA/NCI NIH HHS/United States
- R01 NS064131/NS/NINDS NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- U01 AG006781/NH/NIH HHS/United States
- #I01BX004044/U.S. Department of Veterans Affairs/International
- P30 AG066509/AG/NIA NIH HHS/United States
- I01 BX004044/BX/BLRD VA/United States
- RF1 AG055474/NH/NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- R01 NS064131/NH/NIH HHS/United States
- I01 BX003755/BX/BLRD VA/United States
- #I01BX002619/U.S. Department of Veterans Affairs/International
- UL1 TR000423/TR/NCATS NIH HHS/United States
- RF1 AG055474/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

