Impact of 13-Valent Pneumococcal Conjugate Vaccine on Colonization and Invasive Disease in Cambodian Children
- PMID: 31175819
- PMCID: PMC7145996
- DOI: 10.1093/cid/ciz481
Impact of 13-Valent Pneumococcal Conjugate Vaccine on Colonization and Invasive Disease in Cambodian Children
Abstract
Background: Cambodia introduced the 13-valent pneumococcal conjugate vaccine (PCV13) in January 2015 using a 3 + 0 dosing schedule and no catch-up campaign. We investigated the effects of this introduction on pneumococcal colonization and invasive disease in children aged <5 years.
Methods: There were 6 colonization surveys done between January 2014 and January 2018 in children attending the outpatient department of a nongovernmental pediatric hospital in Siem Reap. Nasopharyngeal swabs were analyzed by phenotypic and genotypic methods to detect pneumococcal serotypes and antimicrobial resistance. Invasive pneumococcal disease (IPD) data for January 2012-December 2018 were retrieved from hospital databases. Pre-PCV IPD data and pre-/post-PCV colonization data were modelled to estimate vaccine effectiveness (VE).
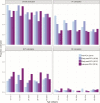
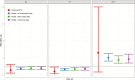
Results: Comparing 2014 with 2016-2018, and using adjusted prevalence ratios, VE estimates for colonization were 16.6% (95% confidence interval [CI] 10.6-21.8) for all pneumococci and 39.2% (95% CI 26.7-46.1) for vaccine serotype (VT) pneumococci. There was a 26.0% (95% CI 17.7-33.0) decrease in multidrug-resistant pneumococcal colonization. The IPD incidence was estimated to have declined by 26.4% (95% CI 14.4-35.8) by 2018, with a decrease of 36.3% (95% CI 23.8-46.9) for VT IPD and an increase of 101.4% (95% CI 62.0-145.4) for non-VT IPD.
Conclusions: Following PCV13 introduction into the Cambodian immunization schedule, there have been declines in VT pneumococcal colonization and disease in children aged <5 years. Modelling of dominant serotype colonization data produced plausible VE estimates.
Keywords: Streptococcus pneumoniae; Cambodia; children; colonization; vaccine.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Assessing the Impact of Pneumococcal Conjugate Vaccines.Clin Infect Dis. 2020 Apr 10;70(8):1589-1590. doi: 10.1093/cid/ciz484. Clin Infect Dis. 2020. PMID: 31175809 No abstract available.
References
-
- Moore MR, Link-Gelles R, Schaffner W, et al. . Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med 2016; 4:399–406. - PubMed
-
- Ladhani SN, Collins S, Djennad A, et al. . Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis 2018; 18:441–51. - PubMed
-
- Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J 2007; 26:123–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

